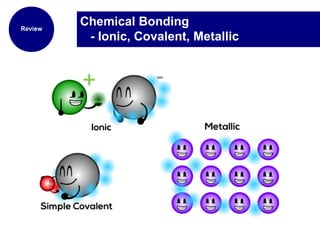
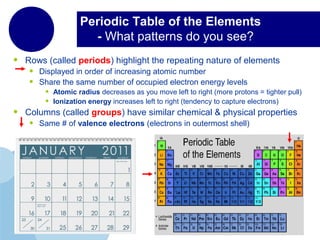
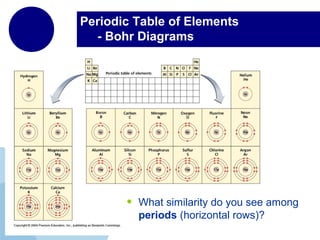
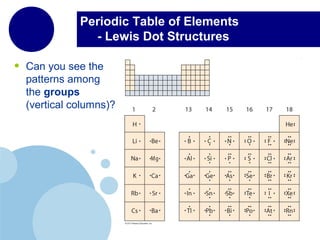
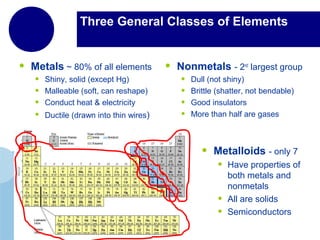
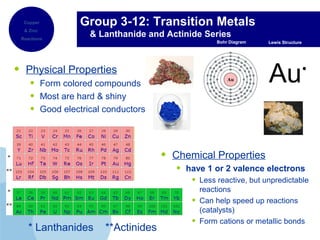
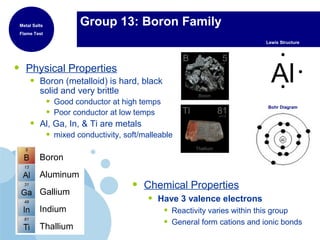
This document provides an overview of the periodic table of elements and key information about each group. It discusses the physical and chemical properties of elements in groups 1-18, including alkali metals, alkaline earth metals, transition metals, and noble gases. Diagrams showing atomic structure (Bohr models and Lewis dot structures) are provided for many elements, along with examples of common compounds and reactions. The periodic trends of atomic radius, ionization energy, and reactivity across periods and down groups are also covered.