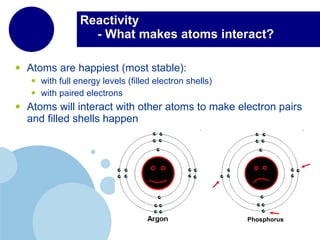
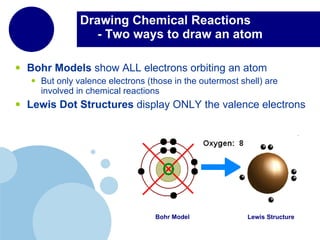
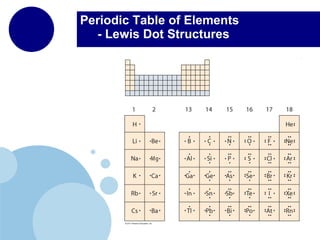
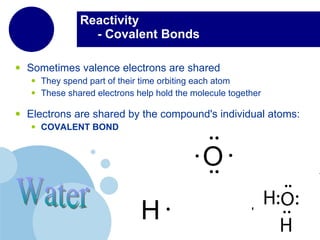
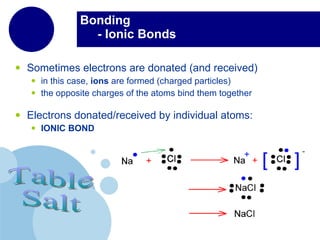
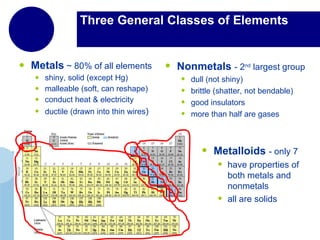
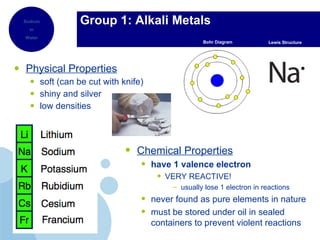
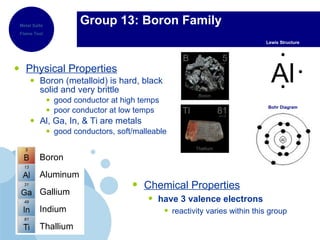
The periodic table organizes elements by atomic number and recurring chemical properties. Elements are grouped into rows called periods and columns called groups based on their atomic structure and how they gain, lose or share electrons to form bonds. The location of an element on the periodic table can provide information about its physical and chemical properties.