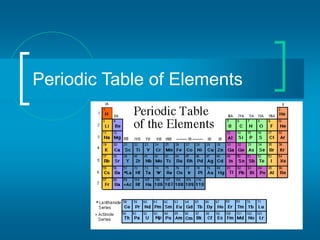
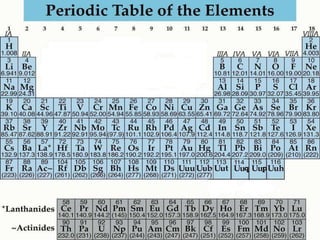
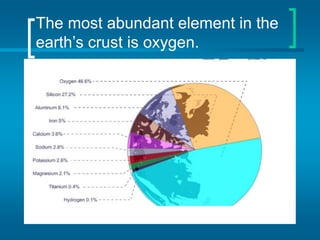
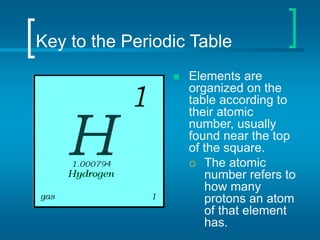
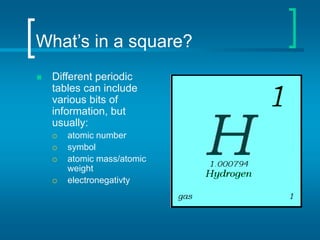
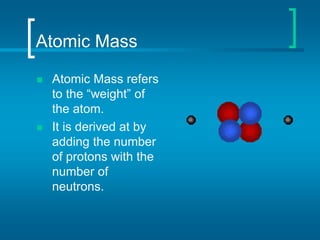
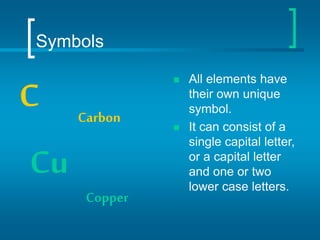
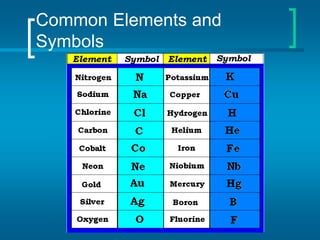
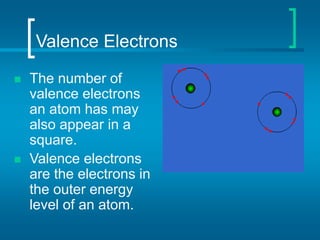
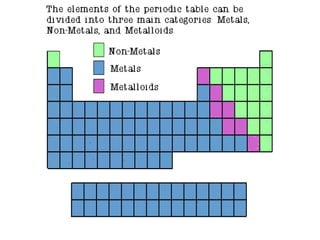
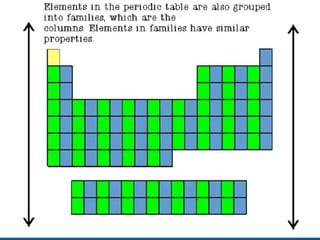
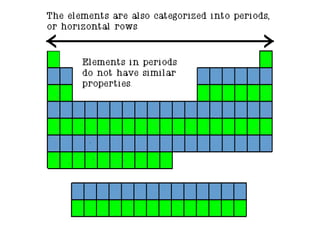
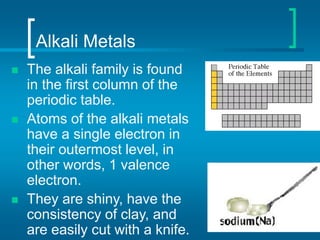
The document provides information about the periodic table of elements, including that it organizes elements according to their atomic number and properties. Key aspects of the periodic table include the atomic number, atomic mass, symbols, and position of elements which can provide insight into their properties. The document also summarizes common elements, families of elements, and properties of metals, non-metals, and metalloids.