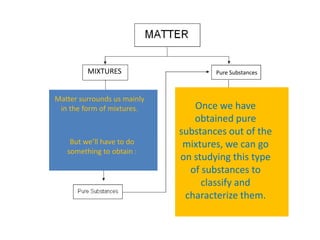
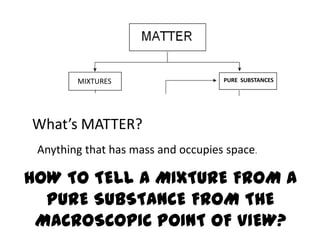
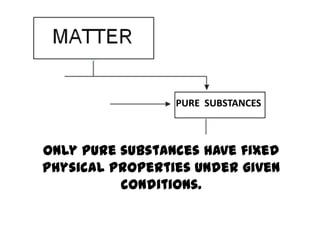
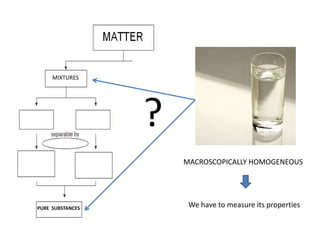
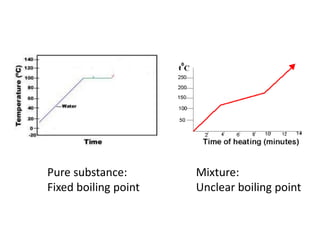
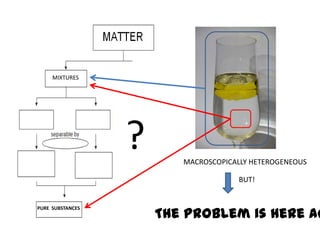
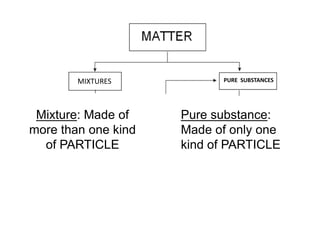
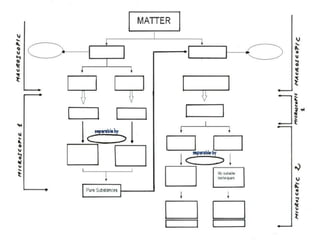
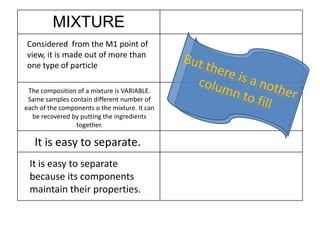
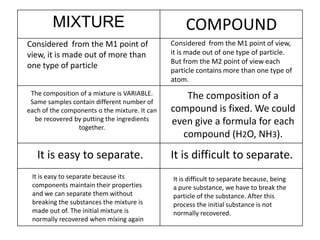
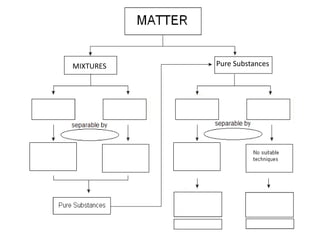
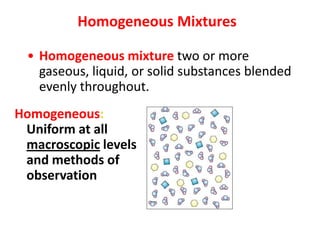
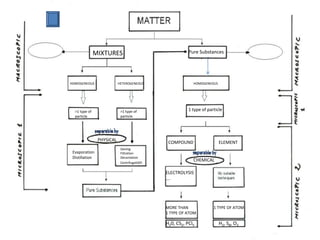
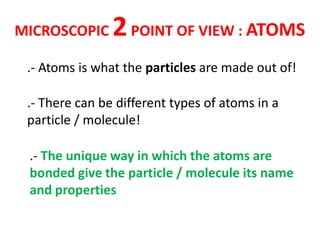
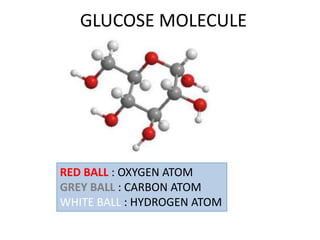
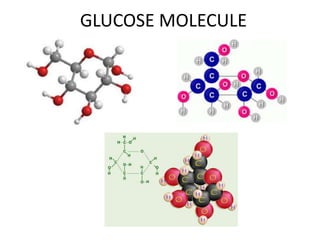
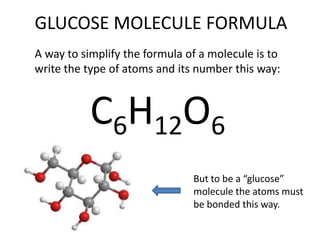
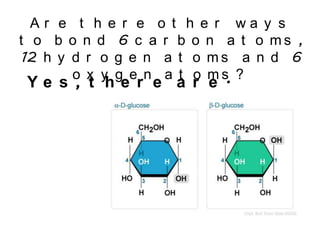
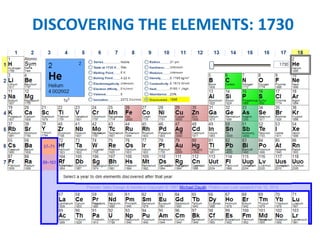
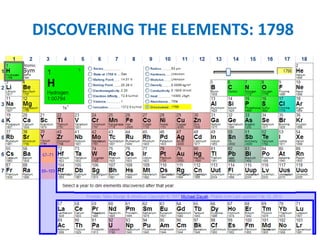
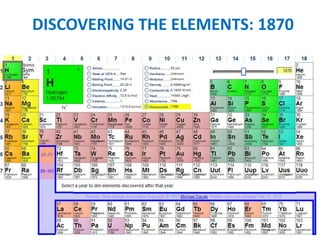
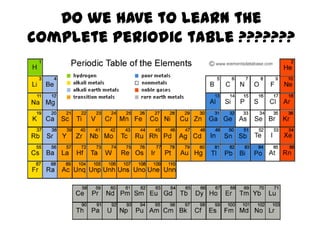
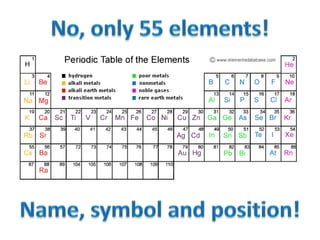
No, you do not need to learn the complete periodic table right away. Focus on learning:
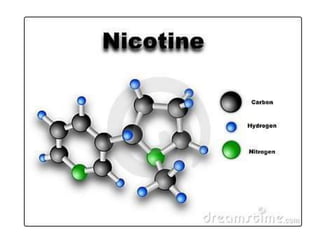
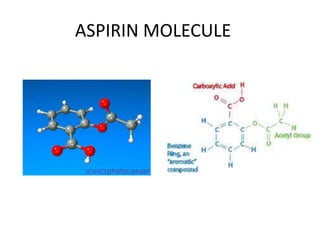
- The names and symbols of the most common elements like H, He, C, N, O, F, Na, Mg, Al, Si, P, S, Cl, K, Ca, etc.
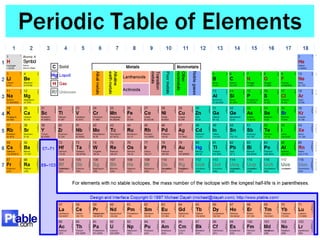
- The overall layout and organization of the periodic table. Pay attention to how elements are grouped based on their properties.
- Key trends as you read across or down the periodic table, such as changes in atomic radius, electronegativity, metallic/non-metallic character.
As you study chemistry concepts involving different elements, you will naturally learn more of the periodic table over time. But it's