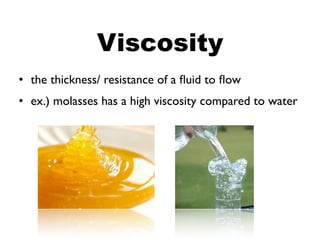
This document discusses the physical and chemical properties of substances. Physical properties can be observed without changing the substance's identity and include properties like state, colour, odour, and hardness. Chemical properties describe a substance's ability to change into new substances through chemical reactions like combustibility, solubility, and reaction with acids. Both qualitative properties observable by the senses and quantitative properties measured with numbers are discussed.