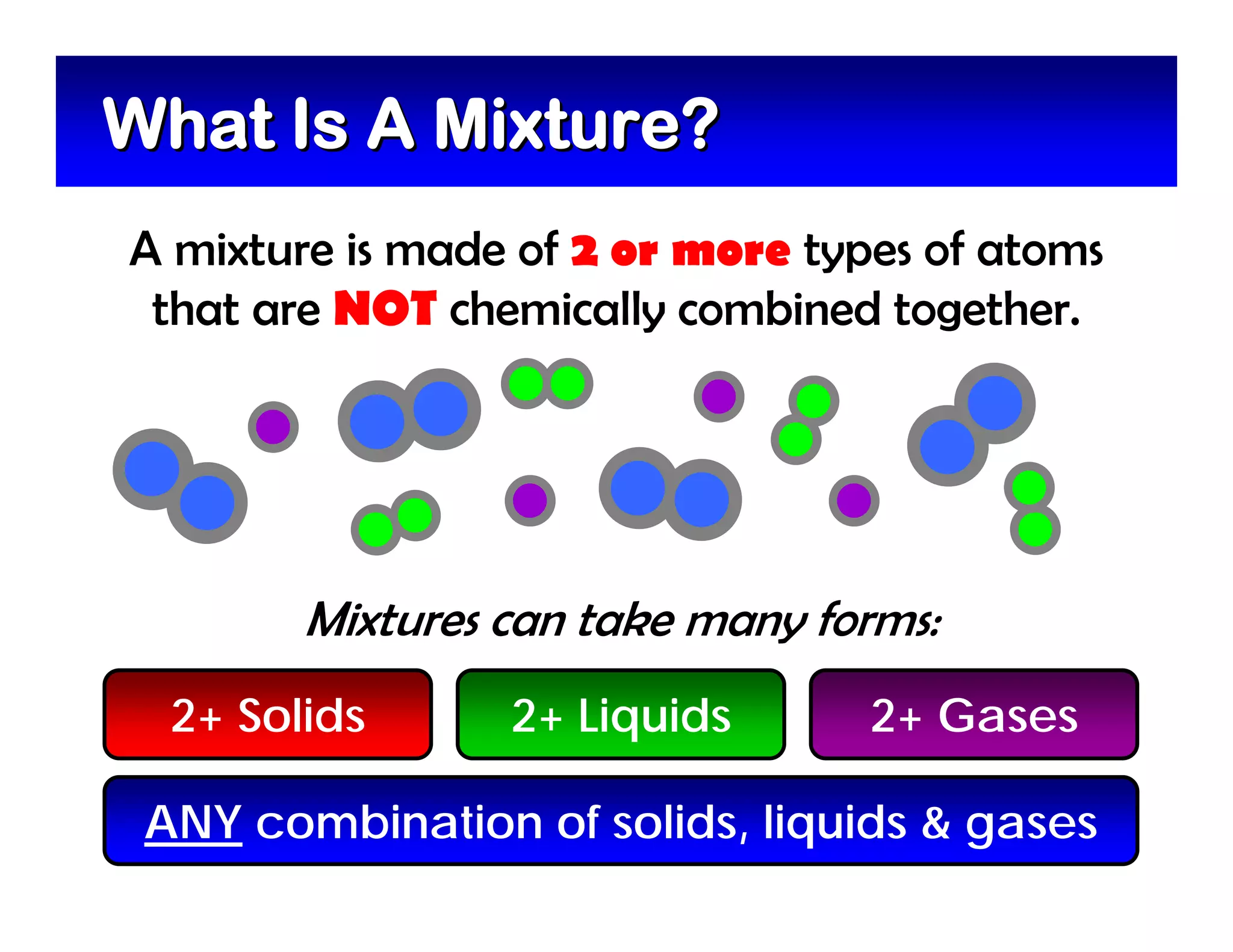
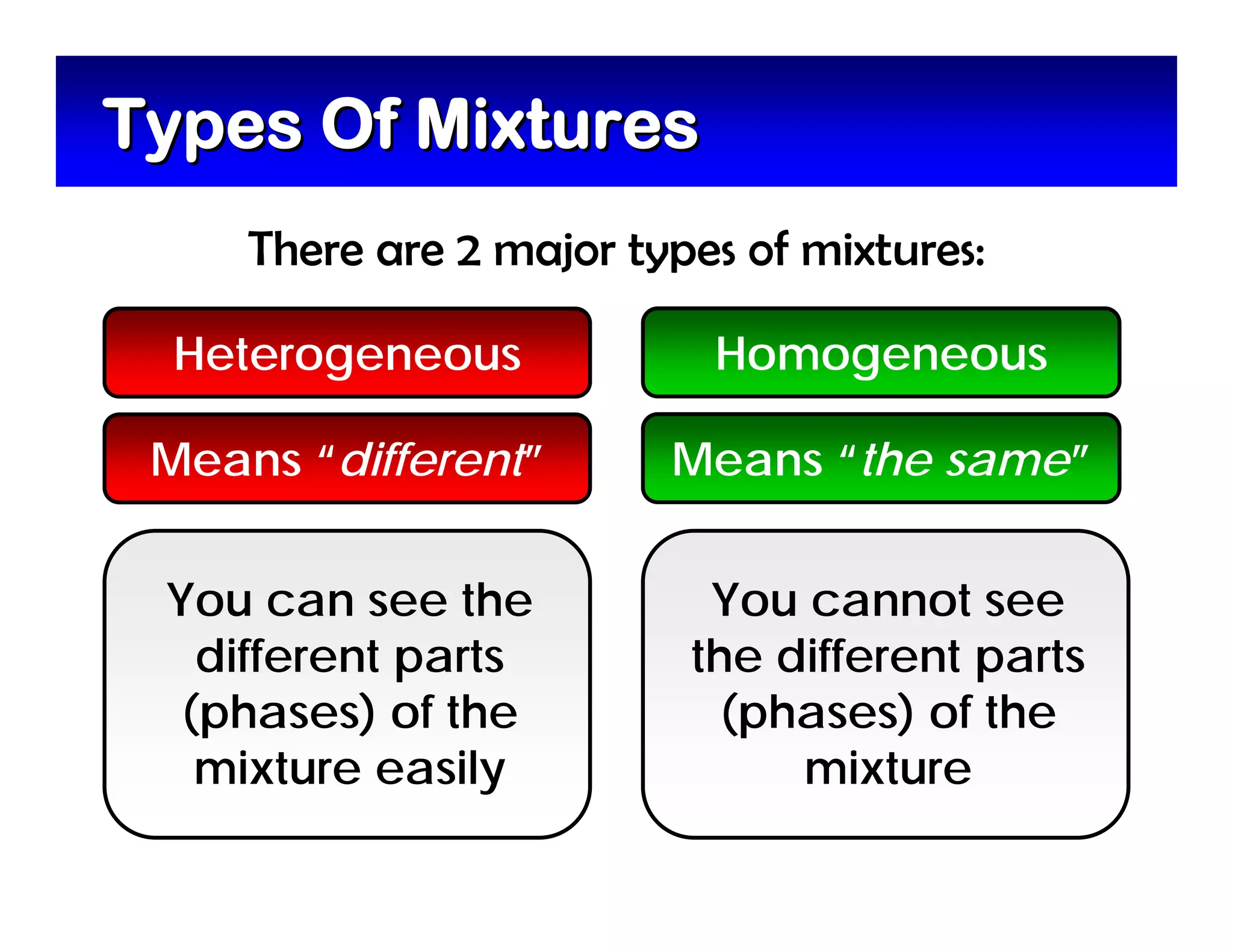
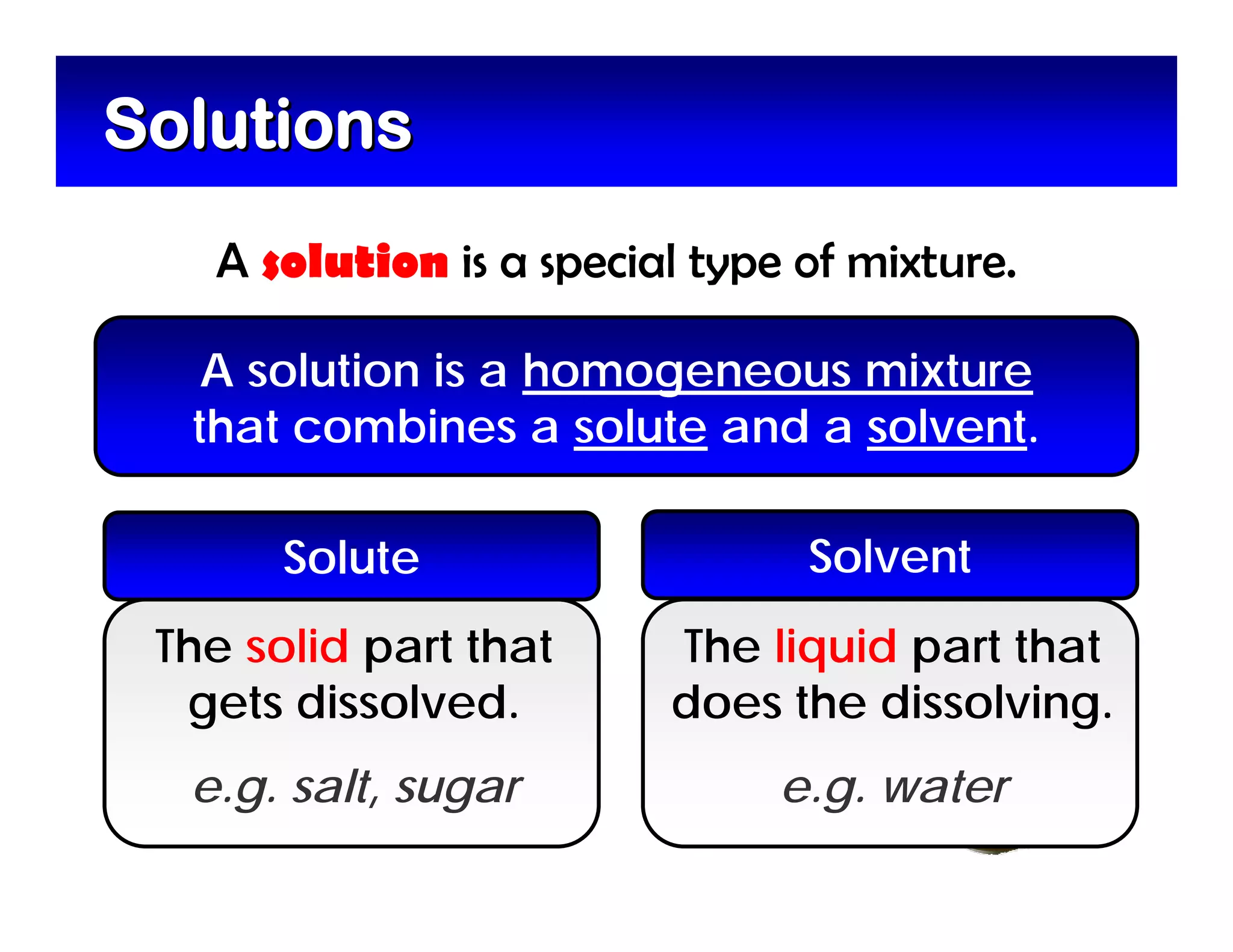
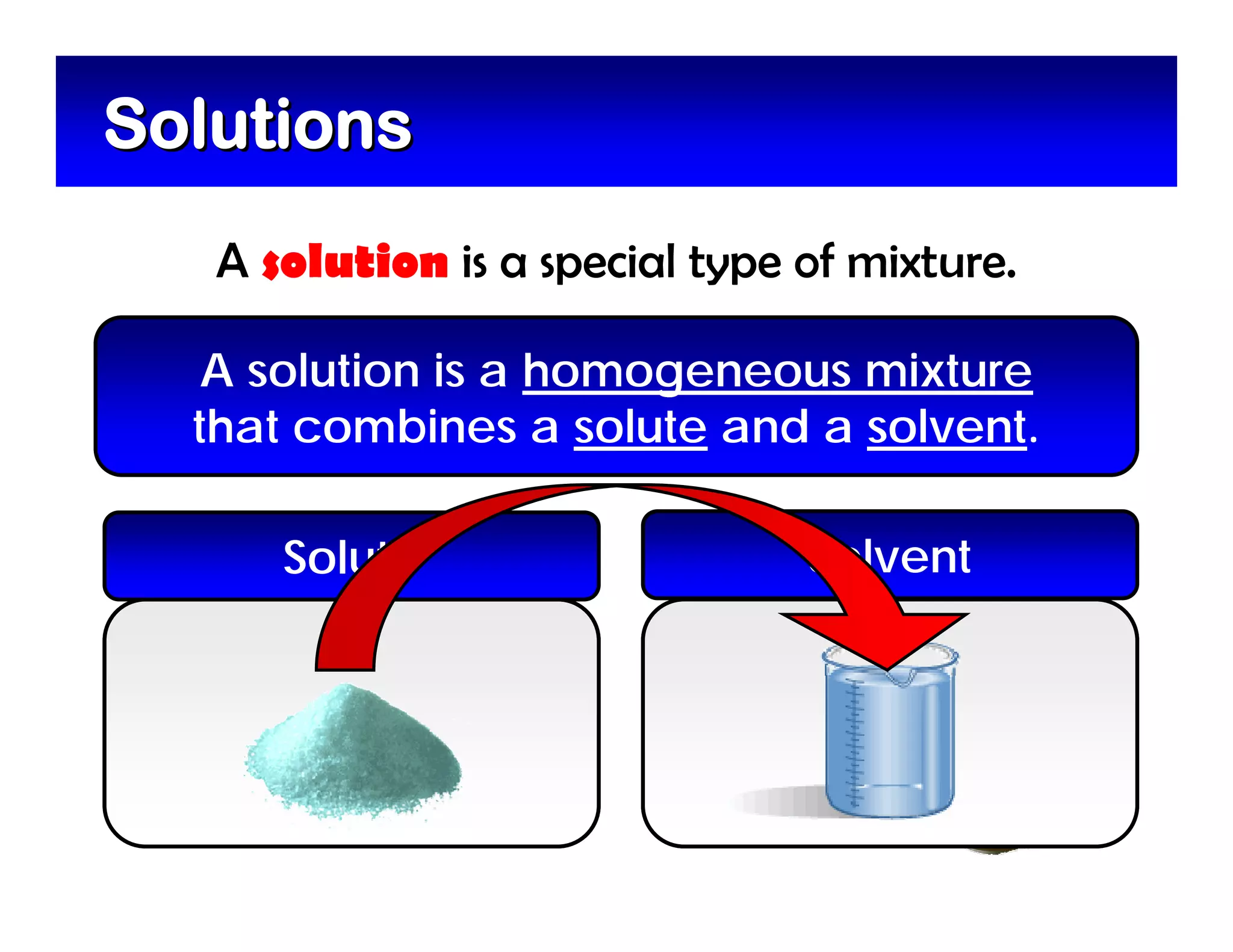
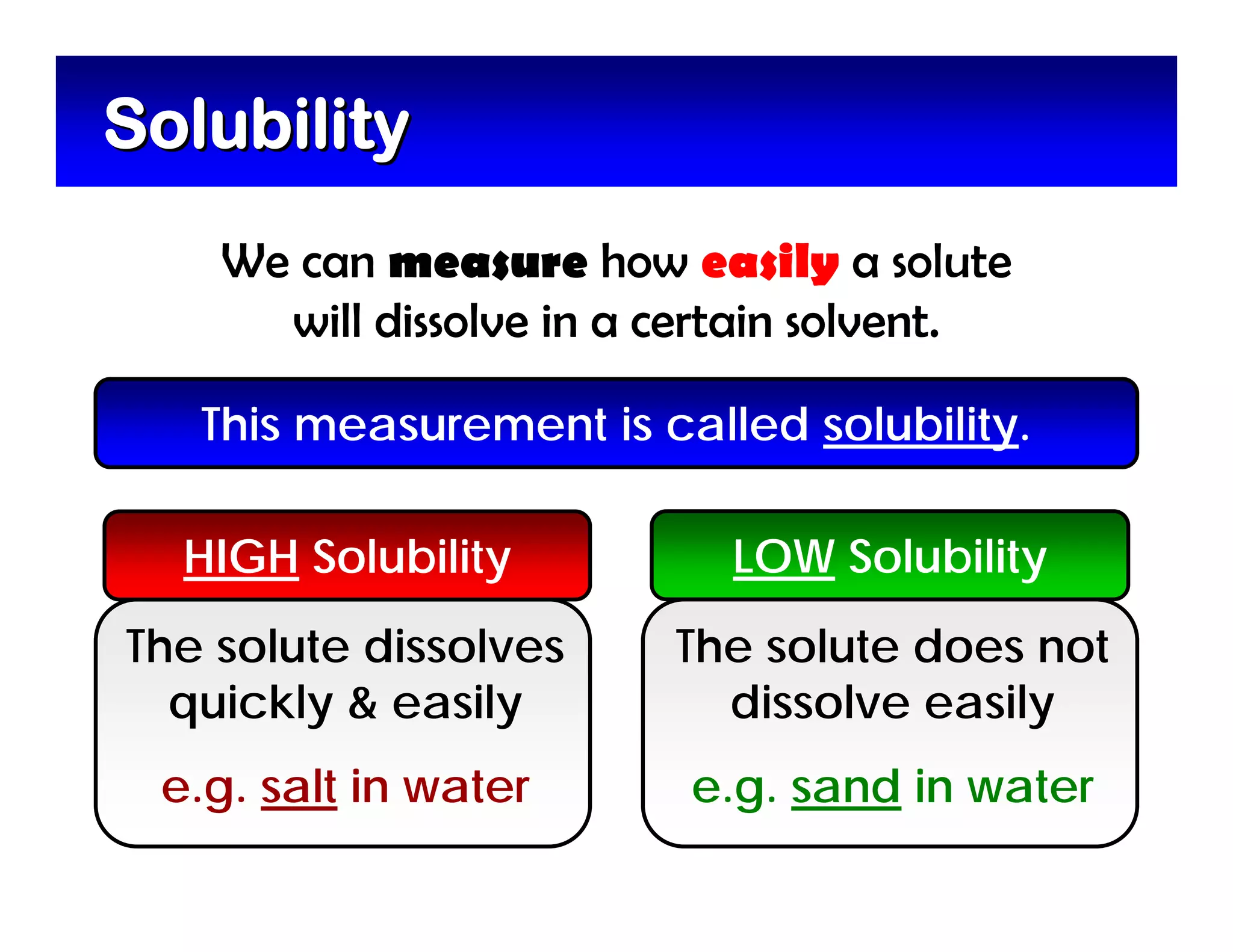
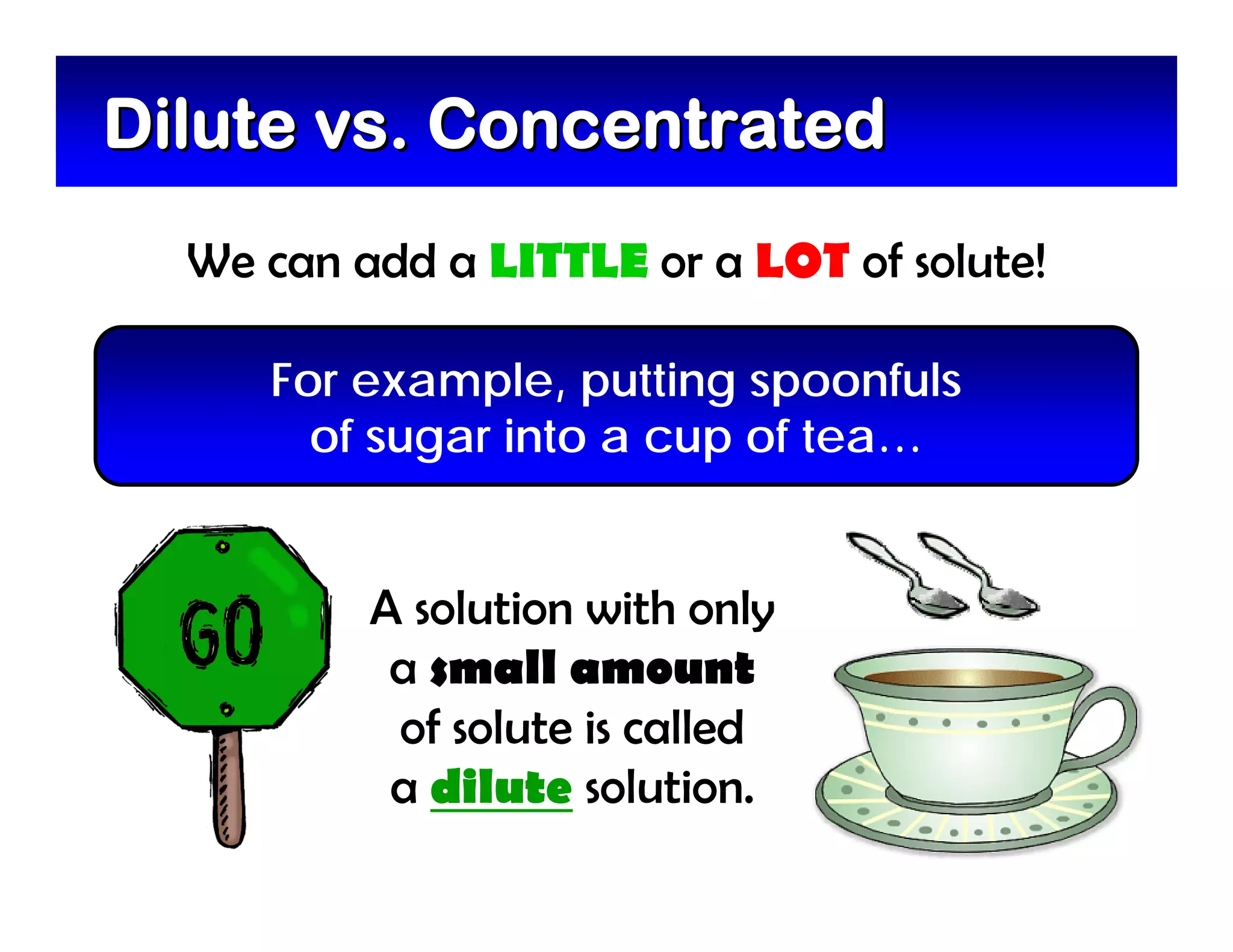
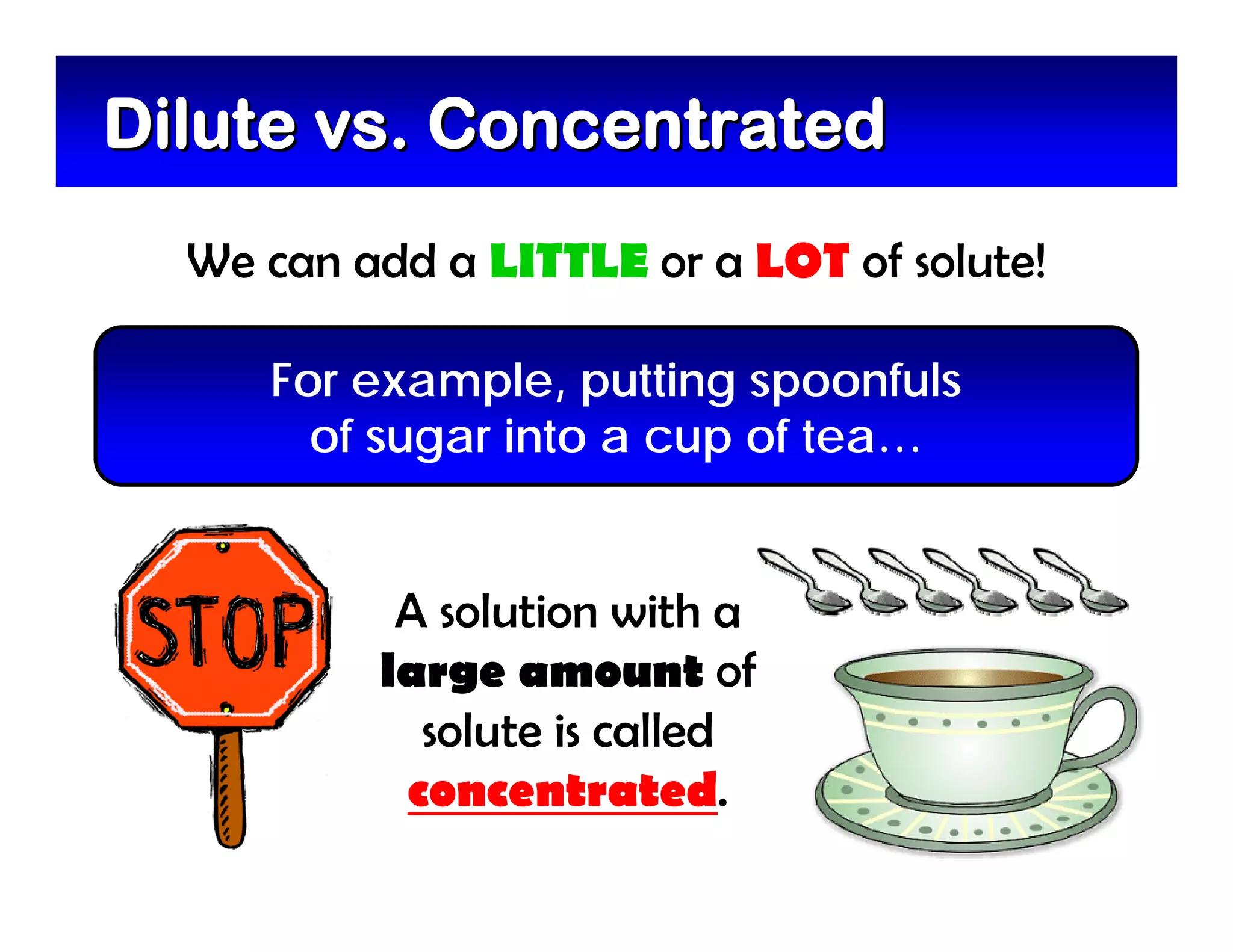
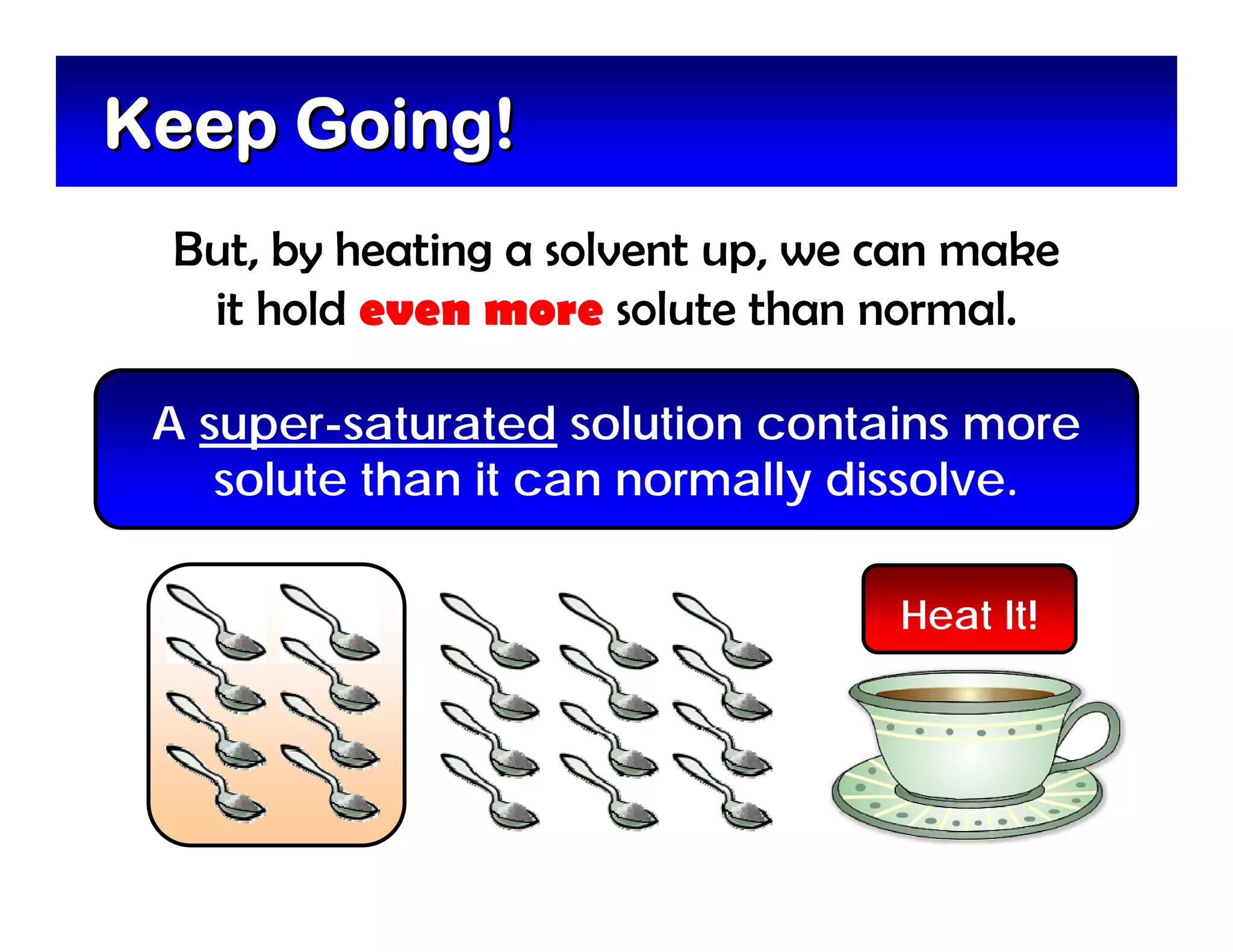
A mixture is composed of two or more substances that are not chemically combined. There are two types of mixtures - heterogeneous mixtures where the phases can be seen, and homogeneous mixtures where the phases cannot be seen. A solution is a special type of homogeneous mixture containing a solute dissolved in a solvent. The solute is the part that dissolves, like salt or sugar, while the solvent does the dissolving and is usually a liquid like water. The concentration of a solution depends on how much solute is added, with dilute solutions having little solute and concentrated solutions having a large amount. A saturated solution contains as much solute as it can dissolve under given conditions, while a supersaturated solution contains more solute than