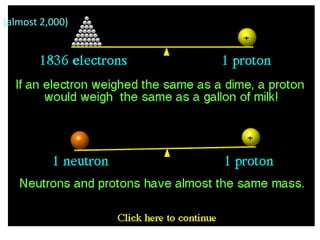
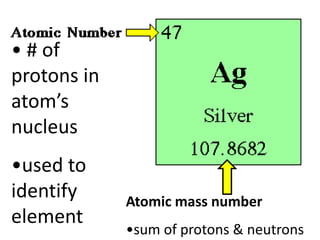
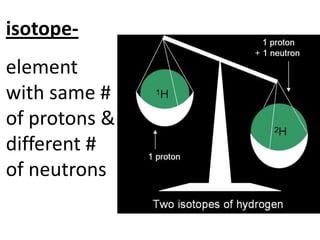
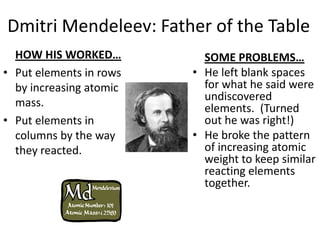
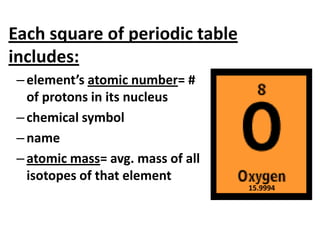
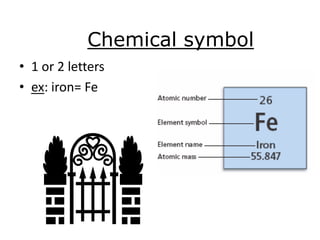
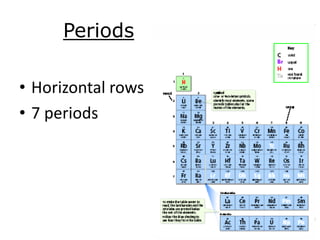
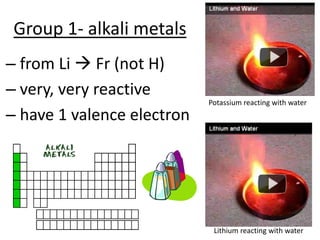
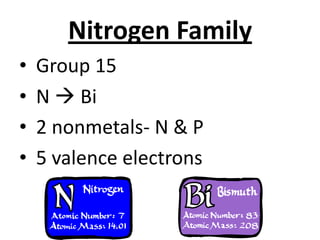
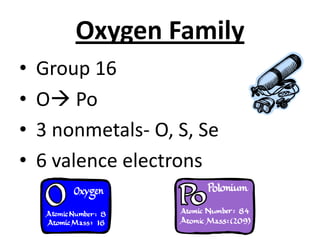
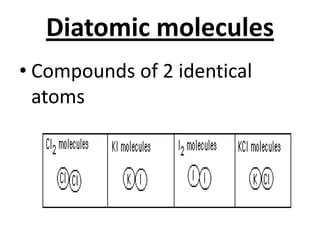
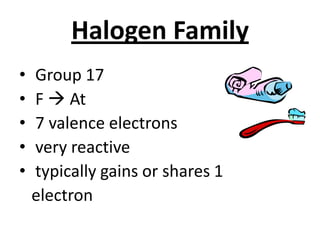
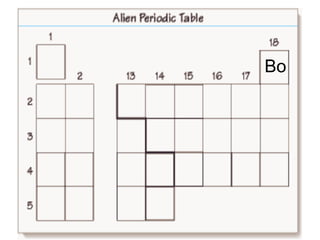
The document discusses the periodic table, including its organization, development, and the information it provides about elements. It explains that the periodic table arranges elements in rows and columns based on atomic number and properties, allowing predictions about undiscovered elements. Each square lists an element's atomic number, symbol, name, and average atomic mass. The periodic table is organized into periods, groups/families that share similar characteristics, and nonmetals, metals and metalloids.