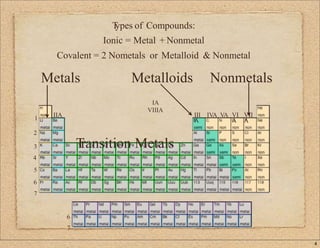
1. The document provides an overview of writing formulas and naming ionic and covalent compounds. It reviews the periodic table and properties of metals, nonmetals and metalloids.
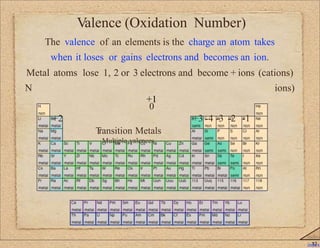
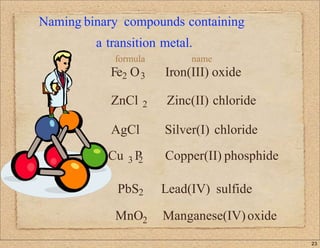
2. Key concepts covered include ion formation, the octet rule, polyatomic ions, oxidation numbers, naming conventions for ionic compounds containing metals or transition metals, and prefixes used in naming covalent compounds.
3. The document distinguishes between ionic and covalent bonding, lattice structures, and molecular structures of compounds.