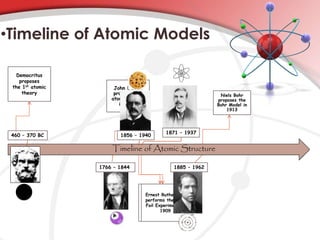
The document summarizes the development of atomic models from Democritus to Bohr. It discusses key contributors including Democritus proposing atoms, Dalton establishing the atomic theory, Thomson proposing the plum pudding model, Rutherford discovering the nucleus through the gold foil experiment, and Bohr refining the model by proposing fixed electron orbits.