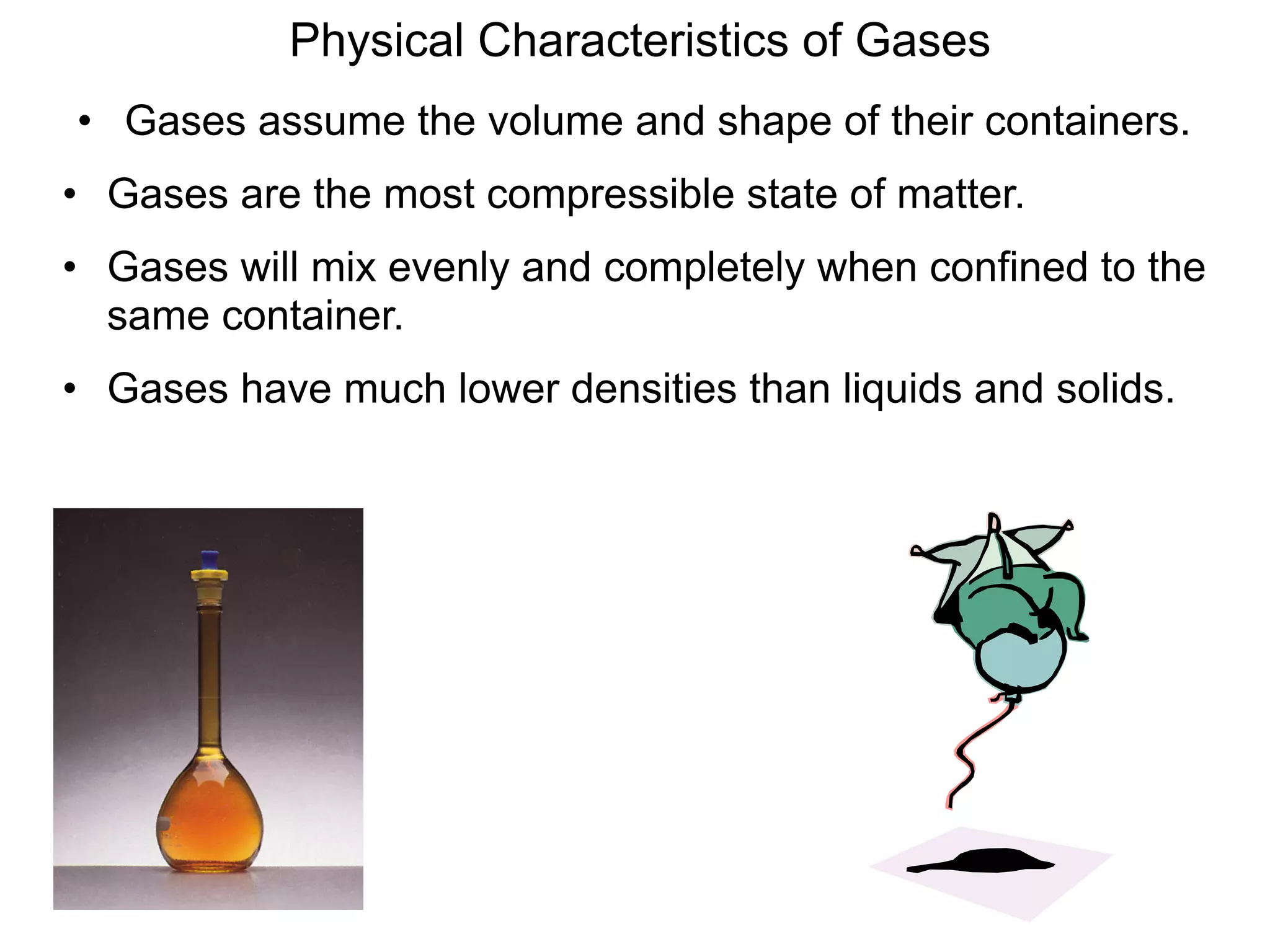
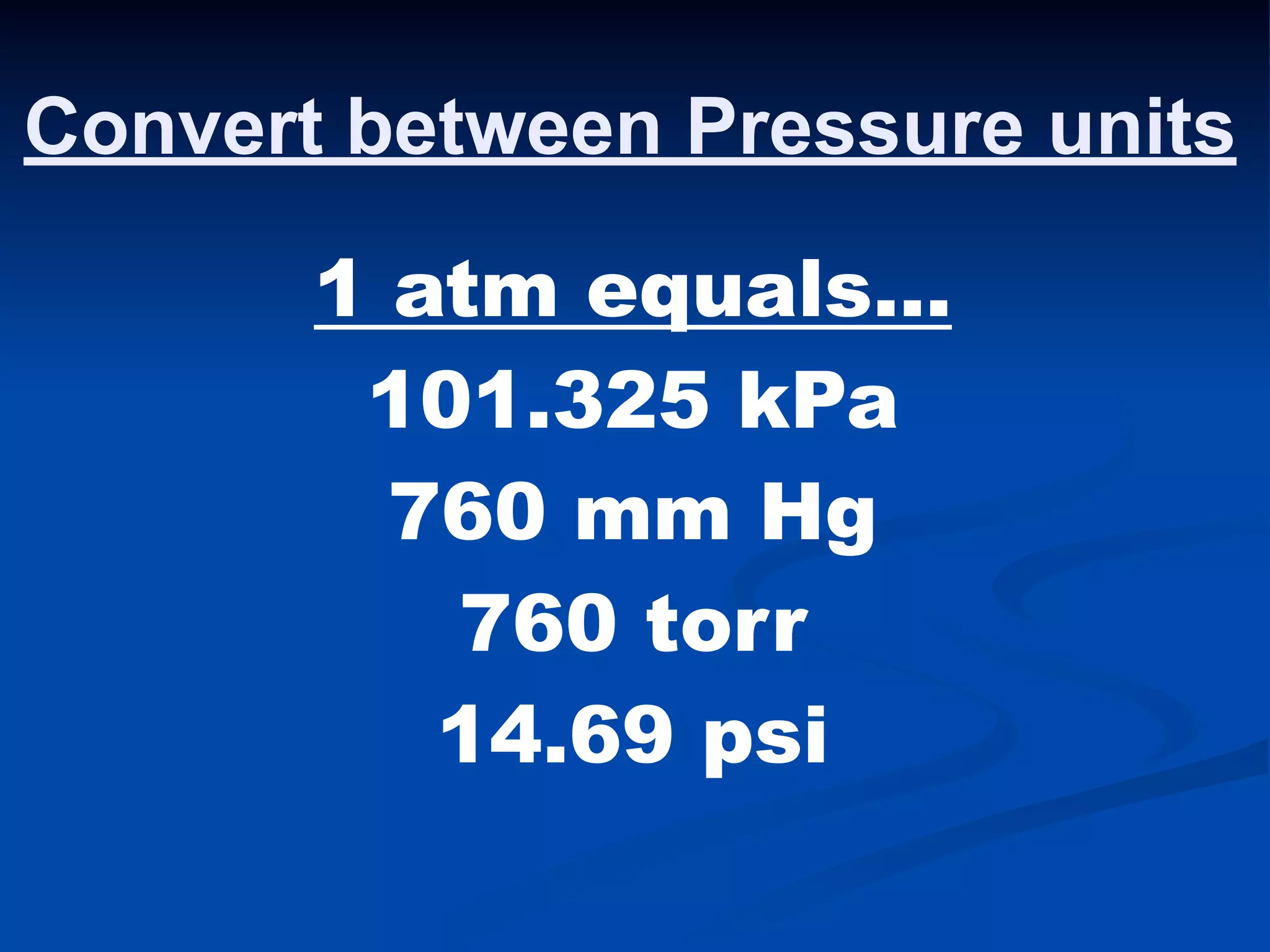
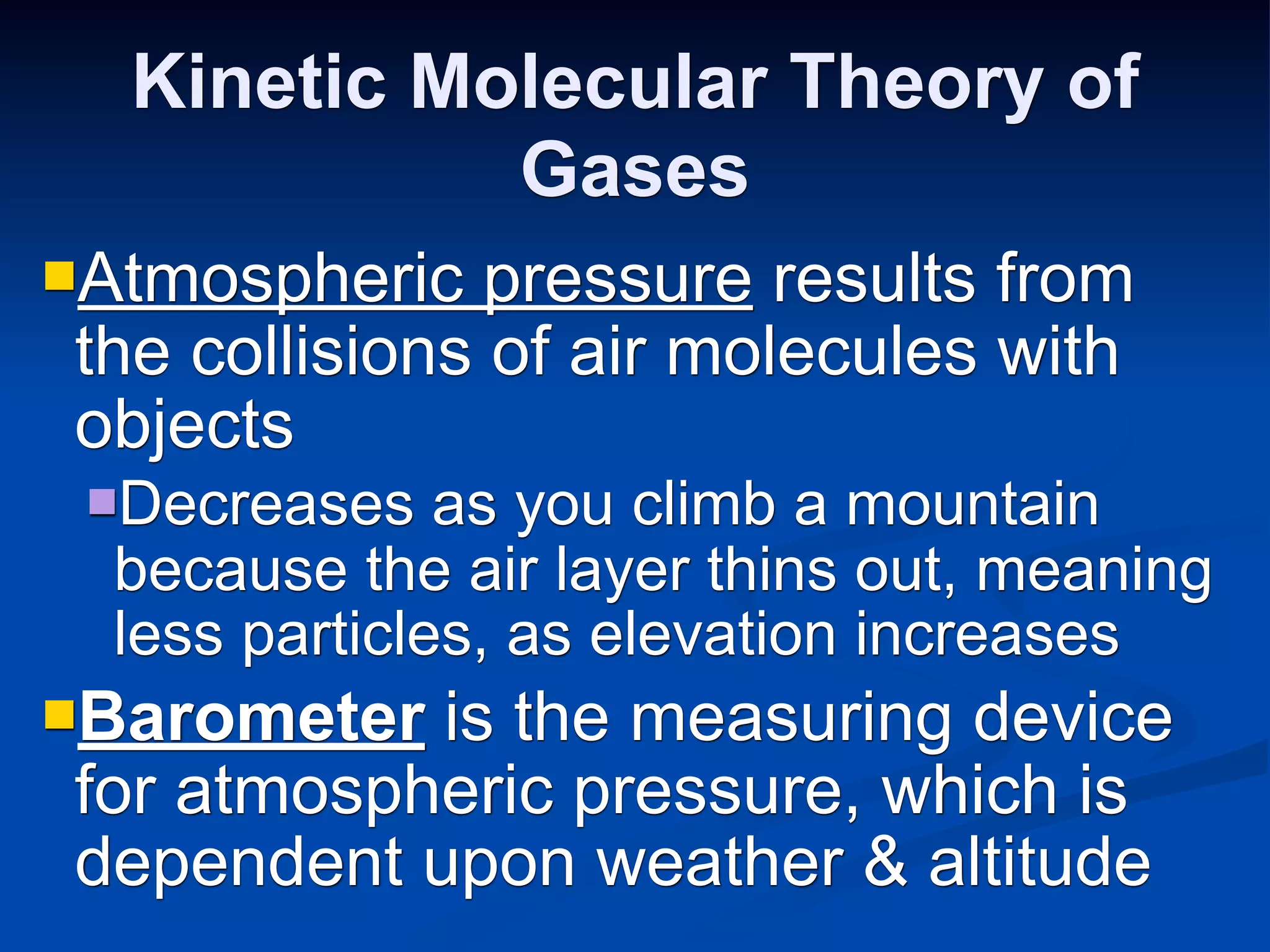
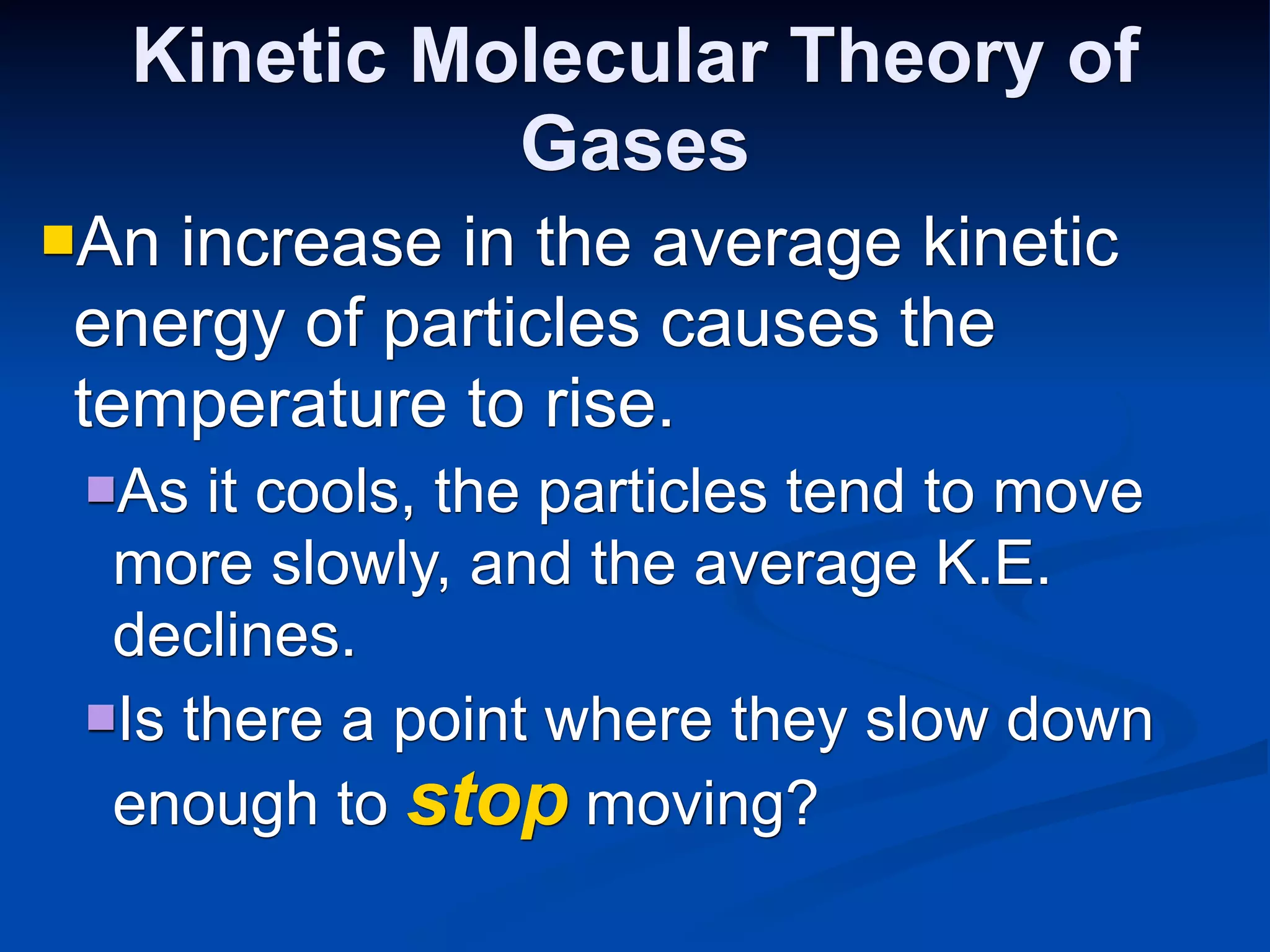
The document discusses the gas laws and properties of gases. It begins by describing the composition of Earth's atmosphere, which is primarily nitrogen and oxygen. It then discusses that gases have mass and low densities compared to liquids and solids. The document outlines four variables that describe gases - pressure, volume, temperature, and amount. It explains concepts such as gas compressibility, units of measurement for gases, and the kinetic molecular theory which describes gas particles as being in constant random motion.