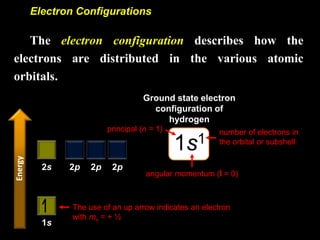
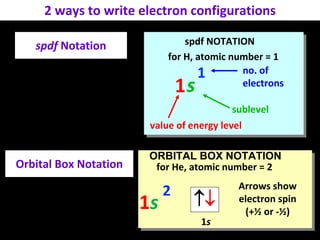
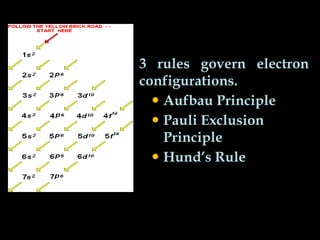
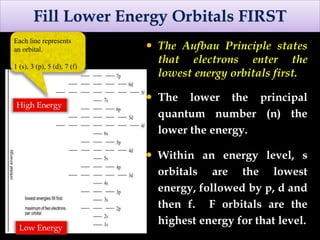
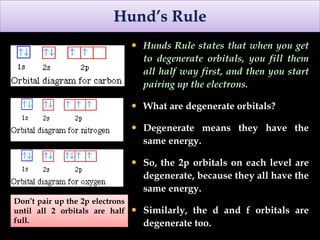
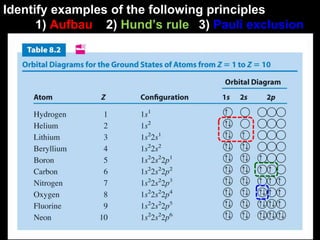
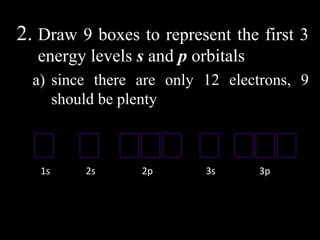
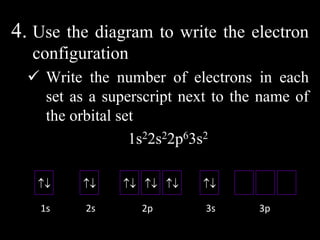
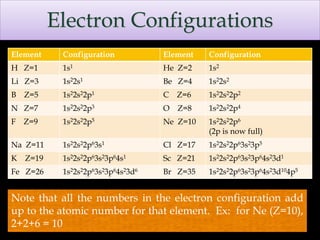
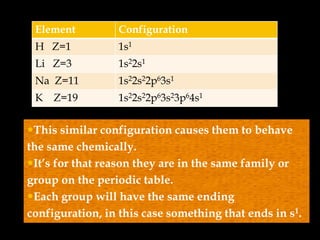
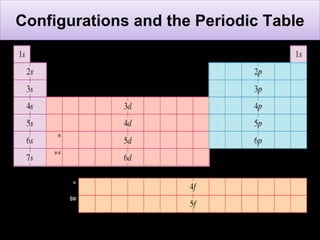
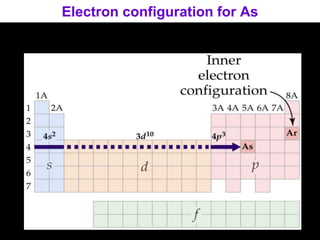
The document discusses electron configurations, which describe how electrons are distributed in atomic orbitals. It explains the Aufbau principle, which states that electrons fill lower energy orbitals first. The Pauli exclusion principle is described, stating that no more than two electrons can occupy any single orbital. Hund's rule is also covered, regarding the filling of degenerate orbitals. Examples are provided to illustrate these principles.









![Shorthand electron configurations
• Because electrons fill orbitals in a regular
pattern, we can shorten the work of writing
electron configurations by using the preceding
noble gas as a template
• We write the highest shell last to indicate the
“valence electrons” - i.e. those furthest out
(involved in bonding and chemical reactions)
• We can represent shorthand electron
configurations of the noble gasses 2 ways: E.g.
Ar = 1s22s22p63s23p6 = [Ne]3s23p6 = [Ar]](https://image.slidesharecdn.com/electronicconfigurationfinal-180210142459/85/Electronic-configuration-final-22-320.jpg)
![• There are several notable exceptions to the order of
electron filling for some of the transition metals.
Chromium (Z = 24) is [Ar]4s13d5 and not [Ar]4s23d4 as
expected.
• Copper (Z = 29) is [Ar]4s13d10 and not [Ar]4s23d9 as
expected.
The reason for these anomalies is the slightly greater
stability of d subshells that are either half-filled (d5) or
completely filled (d10).
4s 3d 3d 3d 3d 3d
[Ar]Cr
Greater stability with half-
filled 3d subshell](https://image.slidesharecdn.com/electronicconfigurationfinal-180210142459/85/Electronic-configuration-final-24-320.jpg)
![• There are several notable exceptions to the order of
electron filling for some of the transition metals.
Chromium (Z = 24) is [Ar]4s13d5 and not [Ar]4s23d4 as
expected.
Copper (Z = 29) is [Ar]4s13d10 and not [Ar]4s23d9 as
expected.
• The reason for these anomalies is the slightly greater
stability of d subshells that are either half-filled (d5) or
completely filled (d10).
4s 3d 3d 3d 3d 3d
[Ar]Cu
Greater stability with filled
3d subshell](https://image.slidesharecdn.com/electronicconfigurationfinal-180210142459/85/Electronic-configuration-final-25-320.jpg)
![Shorthand notation practice
Examples
Aluminum:
Calcium:
Nickel:
Iodine:
Astatine (At):
[Noble Gas Core] + higher energy electrons](https://image.slidesharecdn.com/electronicconfigurationfinal-180210142459/85/Electronic-configuration-final-26-320.jpg)
![Shorthand notation practice
Examples
● Aluminum: 1s22s22p63s23p1
[Ne]3s23p1
● Calcium: 1s22s22p63s23p64s2
[Ar]4s2
● Nickel: 1s22s22p63s23p64s23d8
[Ar]4s23d8 {or [Ar]3d84s2}
● Iodine: [Kr]5s24d105p5 {or [Kr]4d105s25p5}
● Astatine (At): [Xe]6s24f145d106p5
{or [Xe]4f145d106s26p5}
[Noble Gas Core] + higher energy electrons](https://image.slidesharecdn.com/electronicconfigurationfinal-180210142459/85/Electronic-configuration-final-27-320.jpg)





![Practice – Use the Periodic Table to write the short
electron configuration and orbital diagram for each of the
following
• Na (at. no. 11) [Ne]3s1
• Te (at. no. 52) [Kr]5s24d105p4
• Tc (at. no. 43) [Kr]5s24d5
3s
5s 5p4d
5s 4d](https://image.slidesharecdn.com/electronicconfigurationfinal-180210142459/85/Electronic-configuration-final-33-320.jpg)
![Write out the complete electron configuration for the following:
1) An atom of nitrogen
2) An atom of silver
3) An atom of uranium (shorthand)
Fill in the orbital boxes for an atom of nickel (Ni)
2s 2p 3s 3p 4s 3d1s
Which rule states no two electrons can spin the same direction in a single orbital?
1s22s22p3
1s22s22p63s23p64s23d104p65s24d9
[Rn]7s26d15f3
Pauli exclusion principle](https://image.slidesharecdn.com/electronicconfigurationfinal-180210142459/85/Electronic-configuration-final-34-320.jpg)