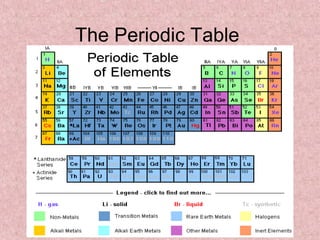
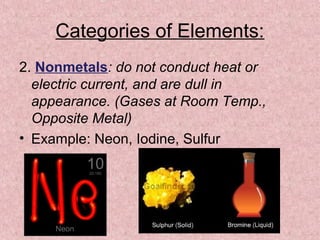
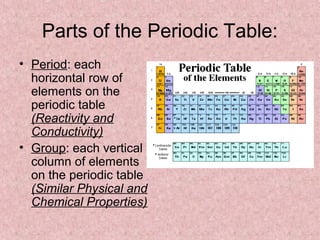
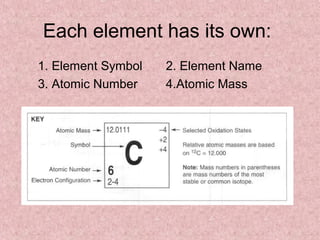
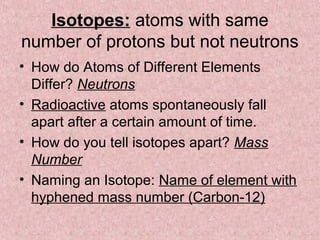
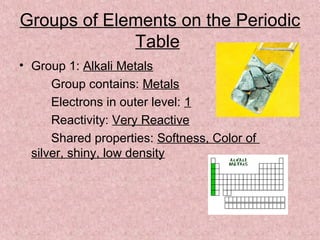
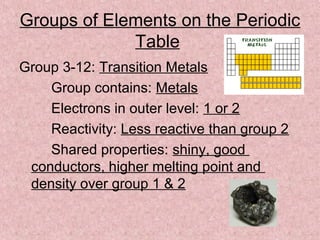
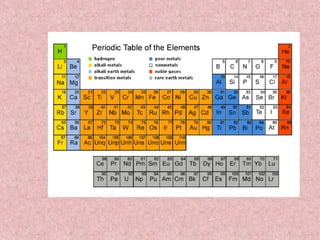
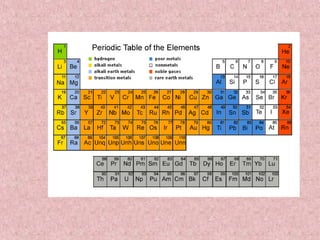
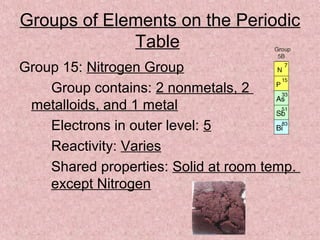
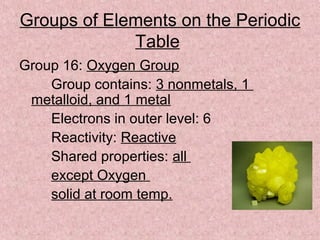
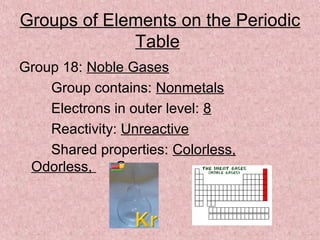
The document summarizes key aspects of the periodic table, including its discovery by Dmitri Mendeleev who predicted undiscovered elements, and the periodic law stating elements' properties repeat periodically with their atomic number. It describes the main categories of elements as metals, nonmetals, and metalloids, and explains parts of the periodic table including periods and groups. It provides details on each group's properties including electron configuration, reactivity, and shared physical traits.