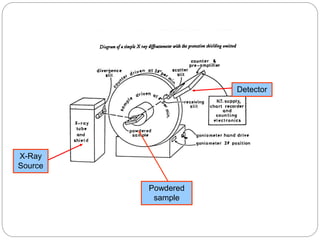
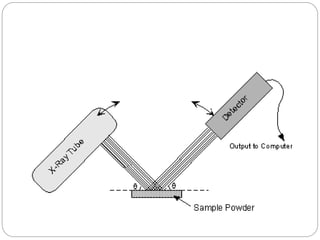
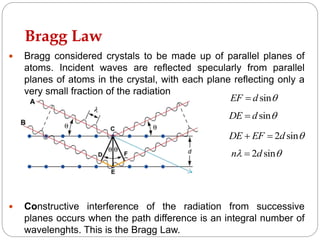
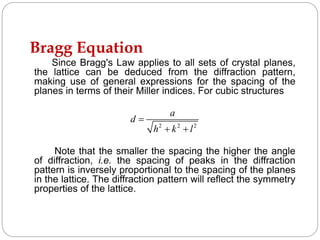
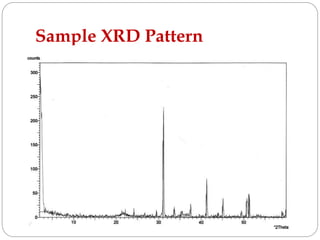
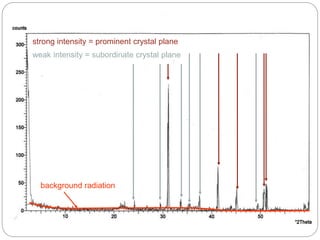
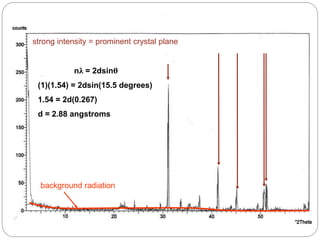
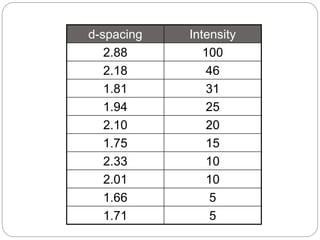
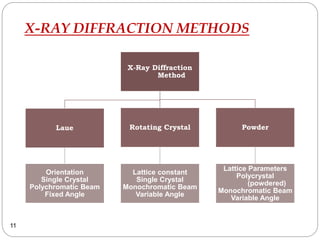
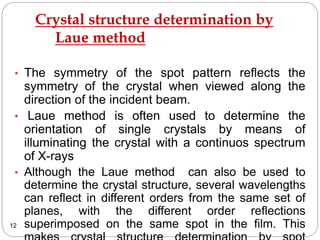
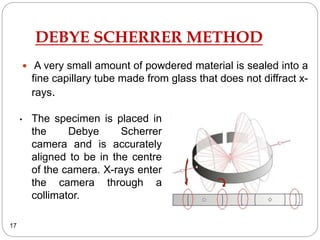
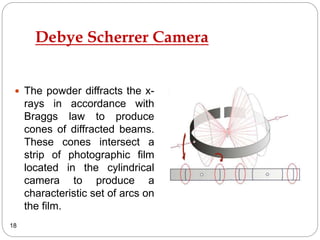
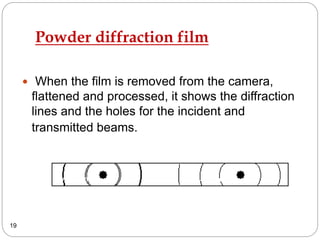
X-ray diffraction is a technique used to analyze the crystal structure of materials. When X-rays strike a crystalline material, they cause the atoms to diffract in predictable patterns. By analyzing these diffraction patterns, properties of the crystal such as its d-spacing and unit cell parameters can be determined. Powder XRD is commonly used, where a sample is finely powdered and exposed to monochromatic X-rays, producing a characteristic diffraction pattern that can identify unknown crystalline materials.