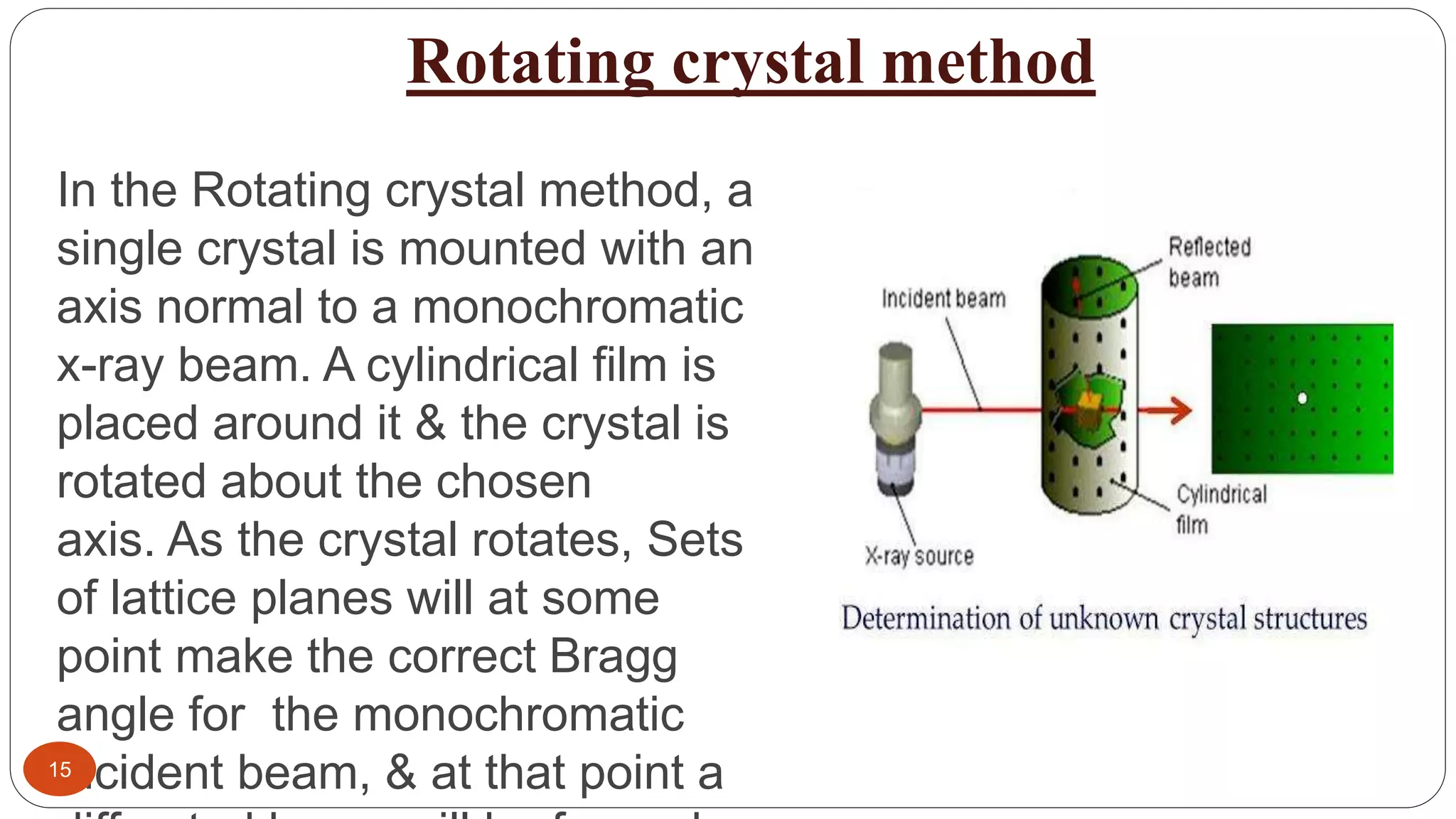
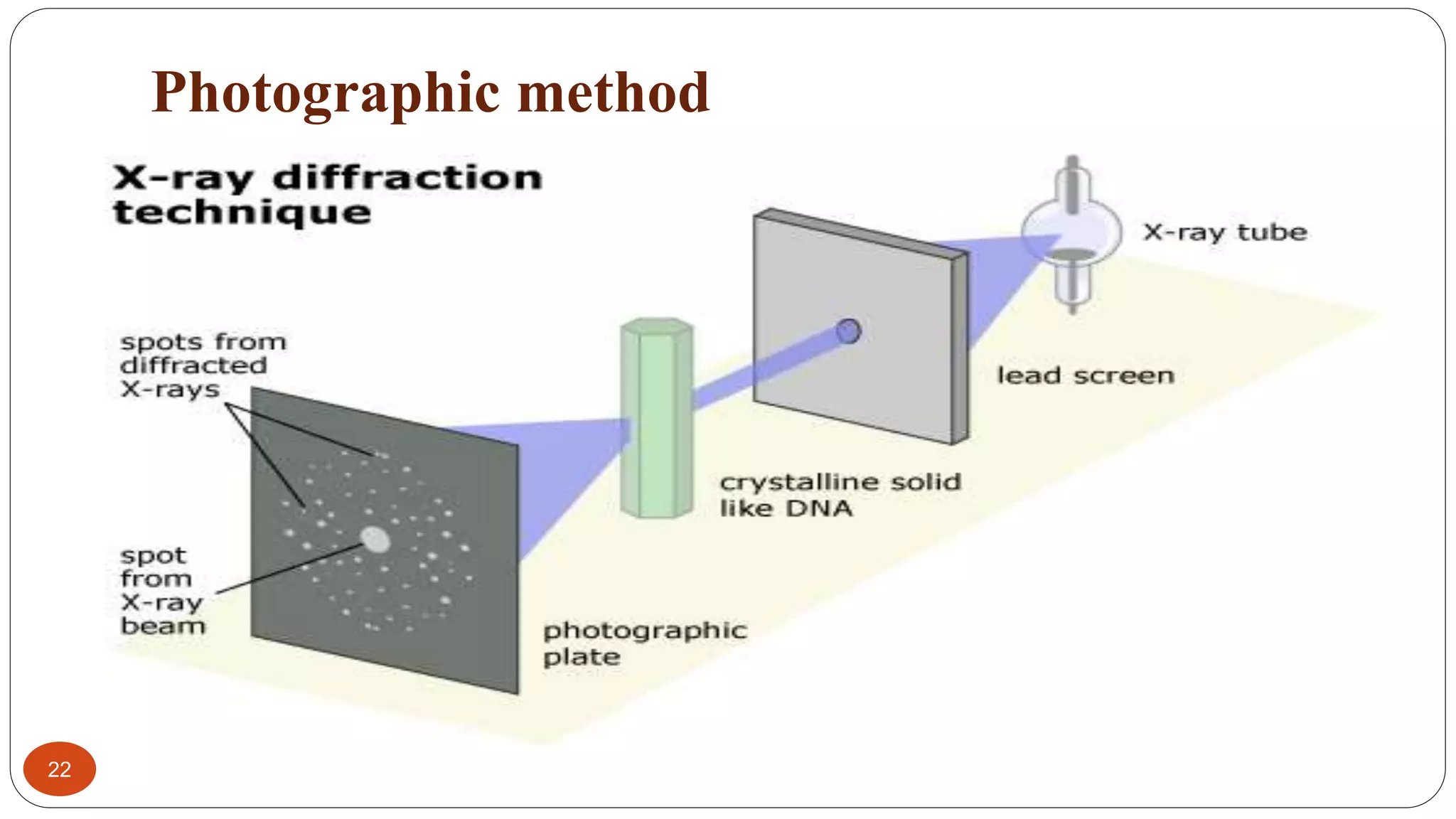
X-ray crystallography uses X-rays to determine the atomic and molecular structure of crystals. Wilhelm Röntgen discovered X-rays in 1895. X-rays are produced when high velocity electrons collide with a metal target. X-ray crystallography works by firing a beam of X-rays at crystalline solids and observing the diffraction pattern of scattered X-rays. Bragg's law describes the conditions under which constructive interference of X-rays occurs and can be used to determine crystal structures. Common methods include rotating crystal, powder diffraction, and using detectors like photographic film, Geiger-Müller counters, or scintillation counters. X-ray crystallography has applications in determining protein structures and identifying