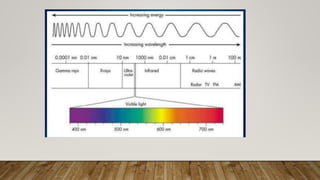
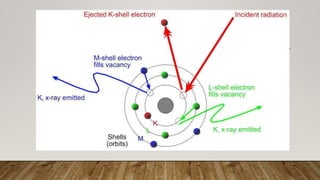
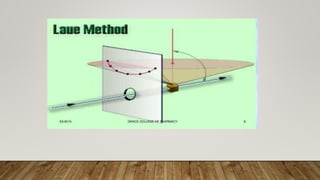
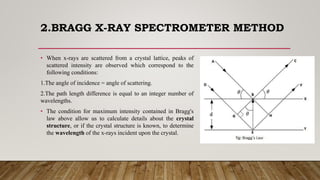
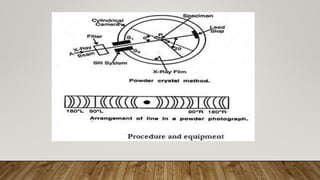
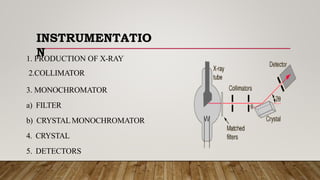
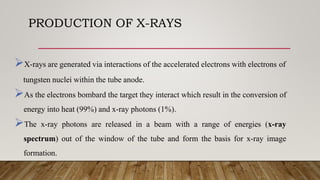
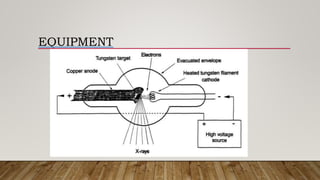
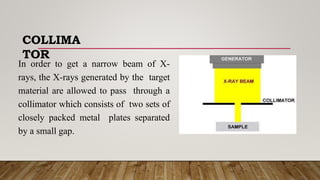
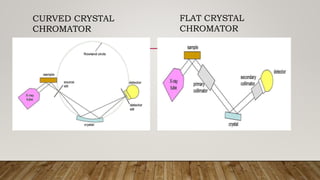
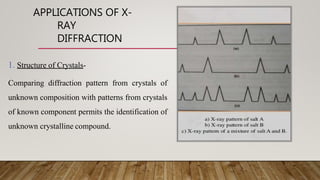
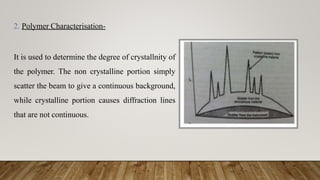
X-ray crystallography uses X-rays to determine the atomic and molecular structure of crystals. When X-rays hit a crystal, they cause the crystalline atoms to diffract the X-rays into specific directions. By measuring the angles and intensities of these diffracted X-rays, the crystallographer can produce a three-dimensional picture of electron density within the crystal. From this electron density, the positions of atoms and chemical bonds in the crystal can be determined. There are several methods for X-ray crystallography including Bragg X-ray spectrometry, rotating crystal method, and powder crystal method. X-ray crystallography has many applications including determining crystal and molecular structures, and character