This document provides an overview of redox reactions including:
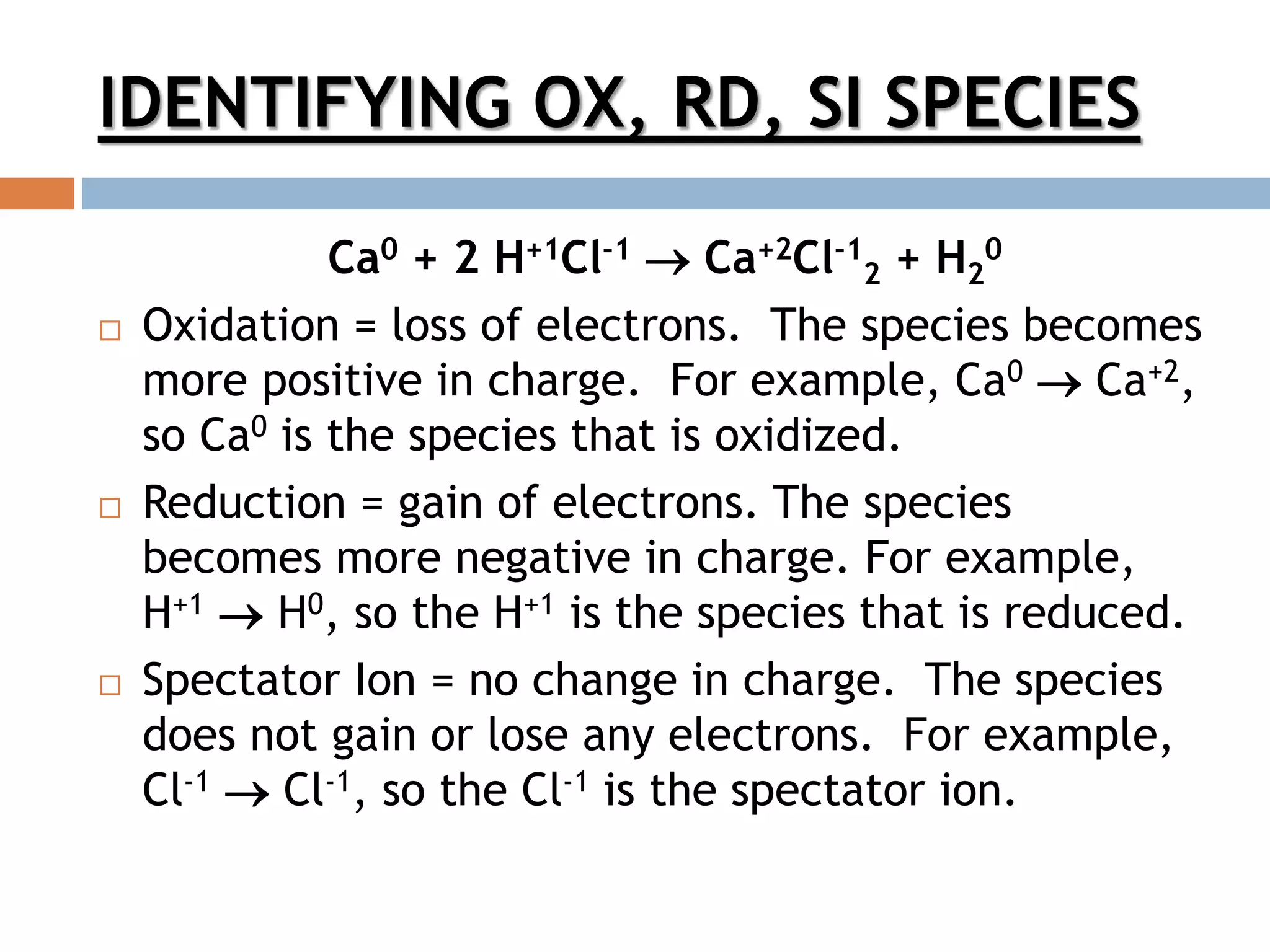
- Redox reactions involve the transfer of electrons between chemical species, resulting in oxidation and reduction.
- Oxidizing agents gain electrons and are reduced, while reducing agents lose electrons and are oxidized.
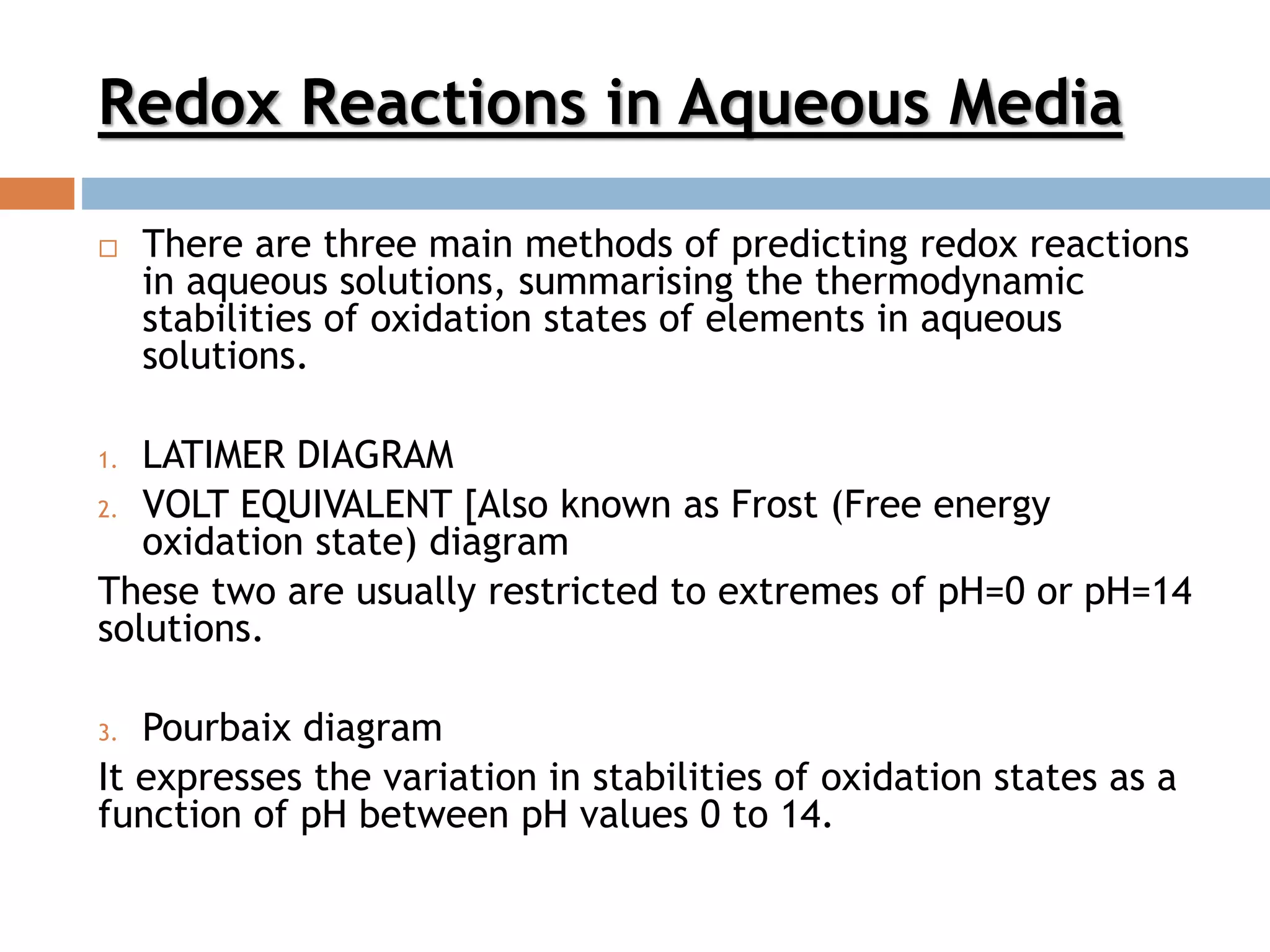
- Latimer, Frost, and Pourbaix diagrams can be used to predict and understand redox reactions in aqueous solutions by showing the thermodynamic stability of different oxidation states.
- Key concepts like disproportionation, oxidizing/reducing abilities, and stable/unstable species can be determined from these types of diagrams.














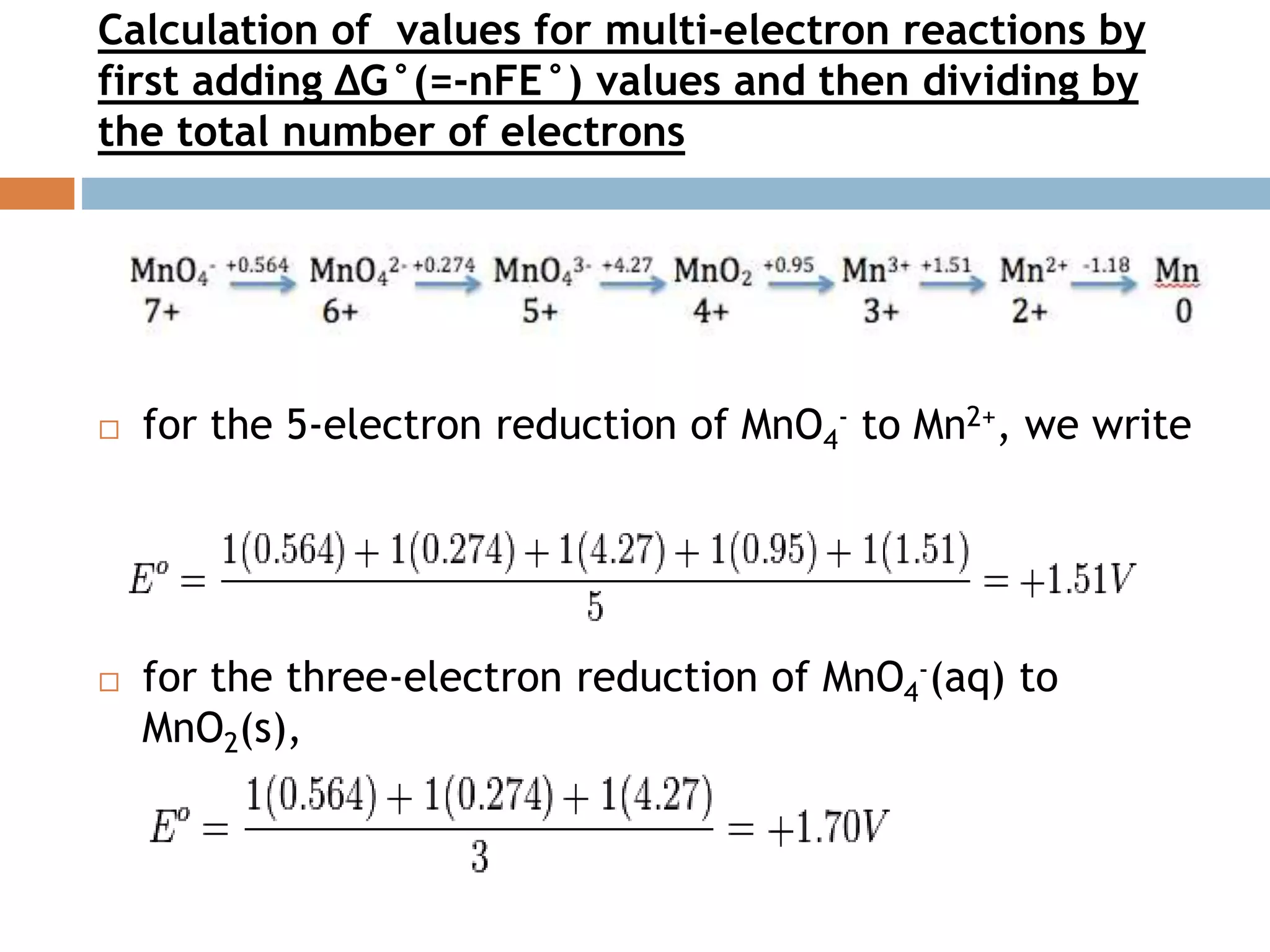
![Latimer diagram for Manganese
[acidic medium]
The Latimer diagram for Mn illustrates its standard
reduction potentials (in 1M HCl) in oxidation states from +7
to 0.
It compresses into shorthand notation all the standard
potentials for redox reactions of element Mn.
Values for multi-electron reactions can be also calculated by
first adding ∆Gº (nFEº) values and then dividing by the total
no of electrons.](https://image.slidesharecdn.com/inorgani-160805152704/75/REDOX-REACTION-17-2048.jpg)