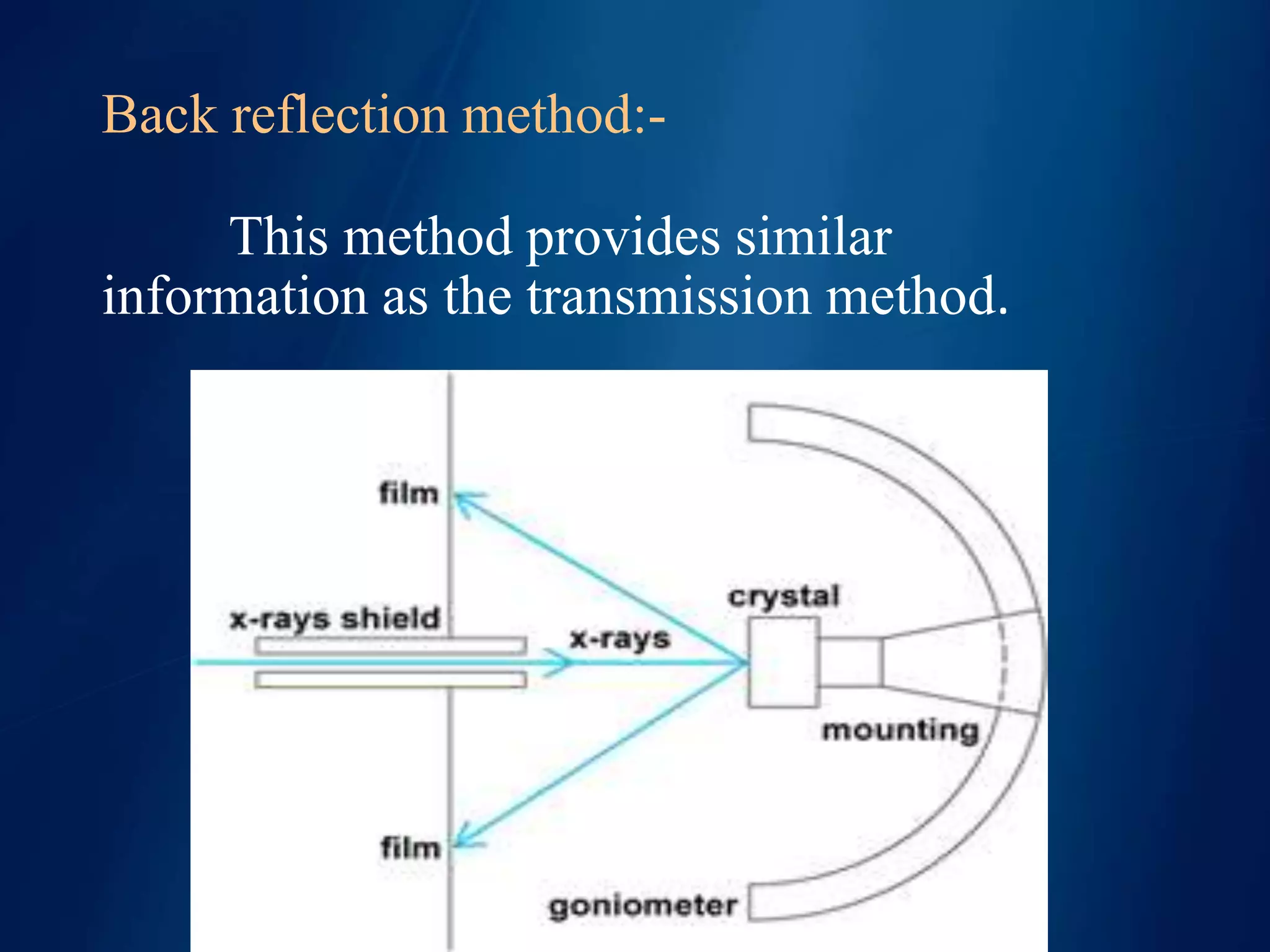
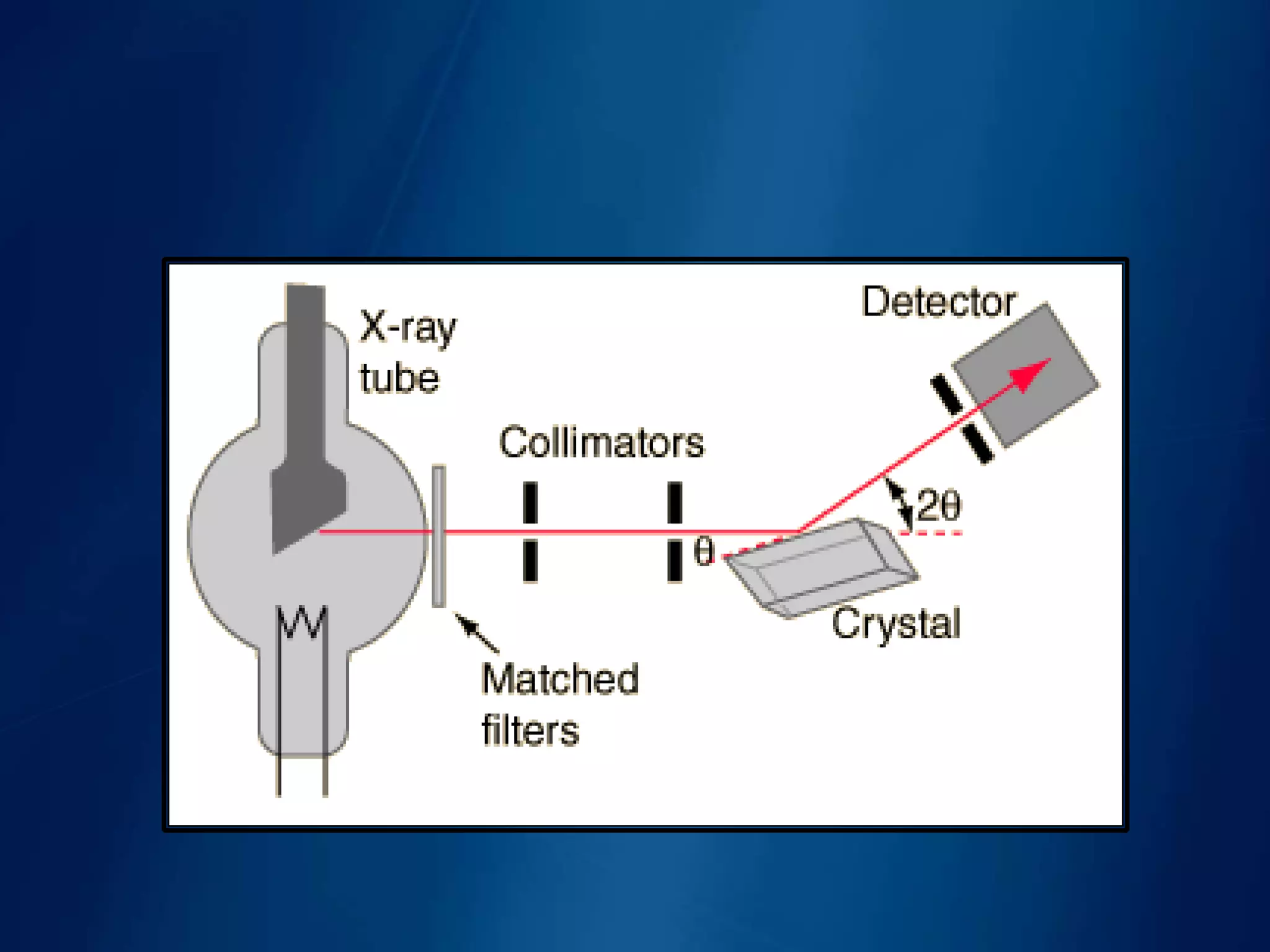
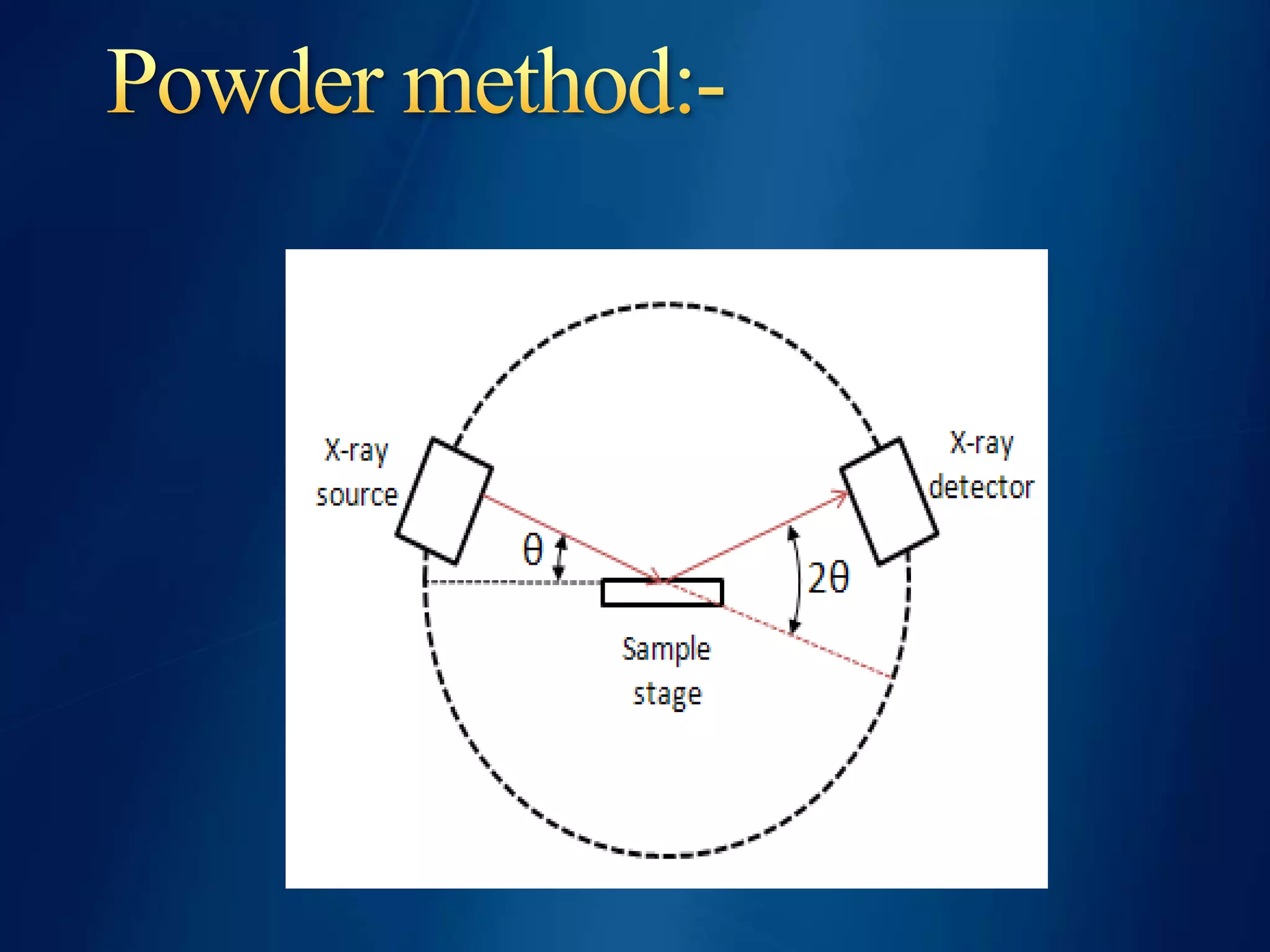
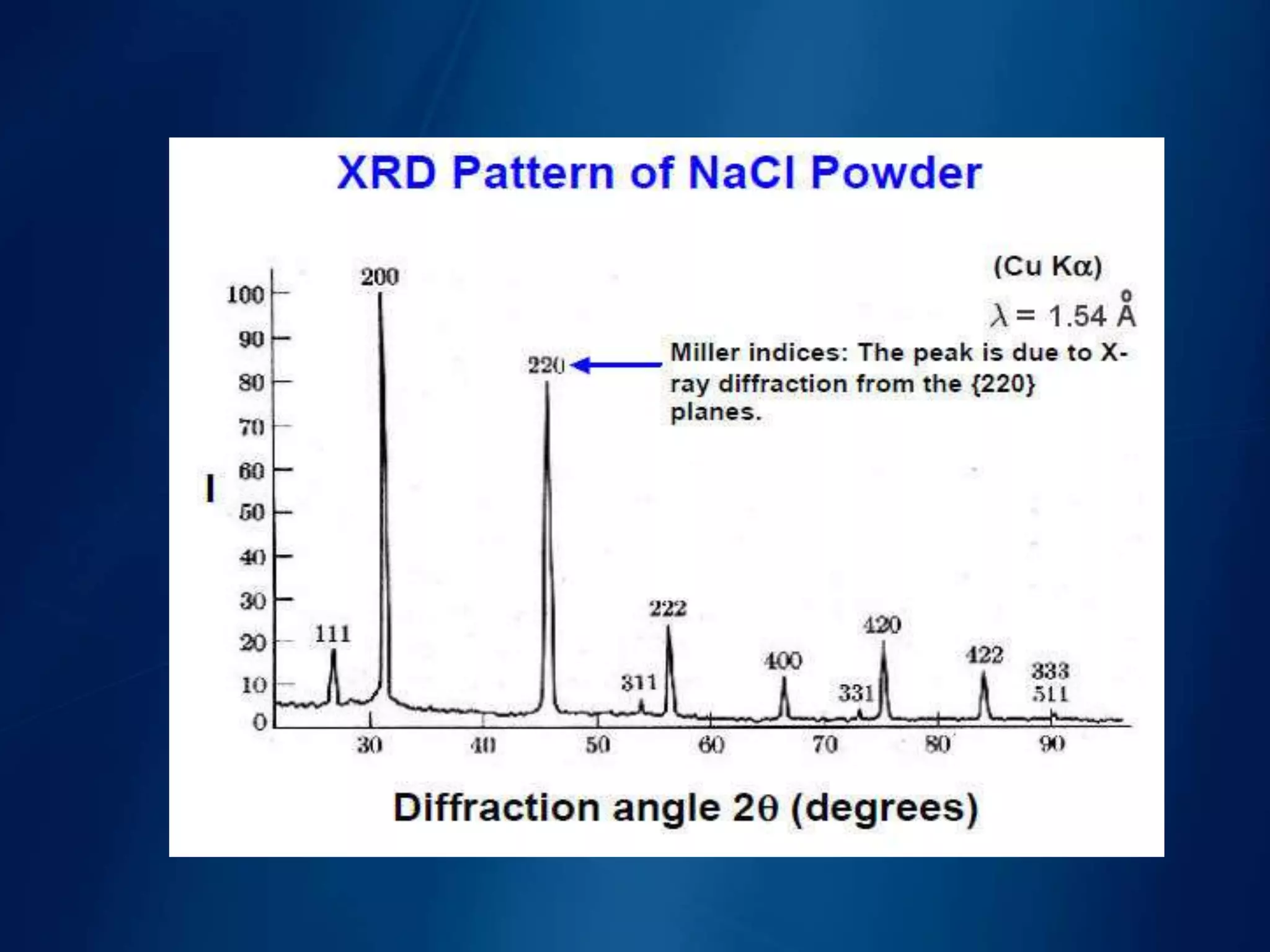
This document discusses X-ray diffraction, including its basic principles and applications. It describes how X-rays are produced via bombardment of a metal target, and how they interact with crystal structures to produce diffraction patterns. Bragg's law is explained as relating the diffraction angle to the wavelength and interplanar spacing. The key methods of X-ray diffraction analysis are powder diffraction and single crystal diffraction using Bragg spectrometers or rotating crystal cameras. Its applications include mineral identification, crystalline structure determination, and measurement of crystallite size.