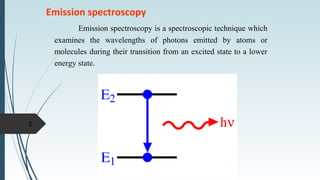
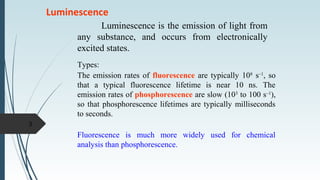
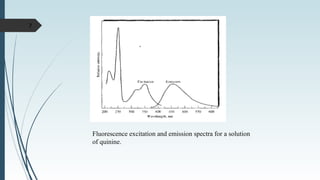
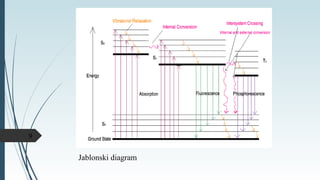
Fluorescence spectroscopy involves using ultraviolet light to excite electrons in molecules, causing them to emit visible light. The emitted light has a longer wavelength than the absorbed light. Fluorimeters are used to measure fluorescence, exciting samples at an absorption wavelength and measuring emission at a longer fluorescence wavelength. Fluorescence spectroscopy is useful for applications like determining fluorescent drugs in formulations, carrying out limit tests for fluorescent impurities, and studying drug-protein binding in bioanalysis.