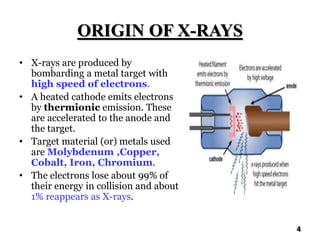
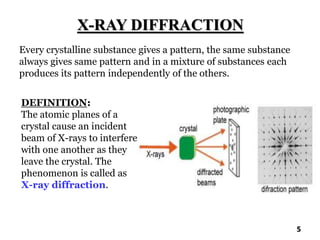
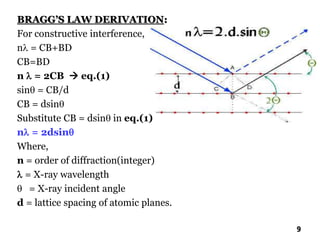
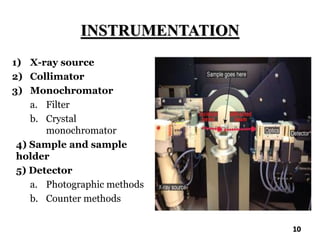
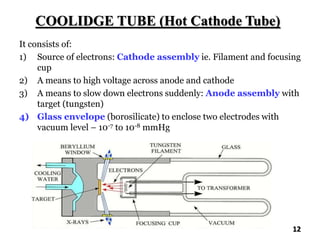
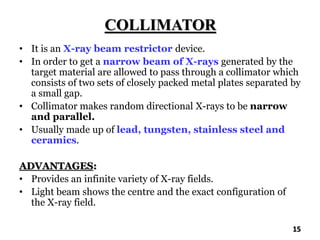
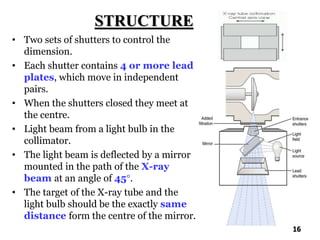
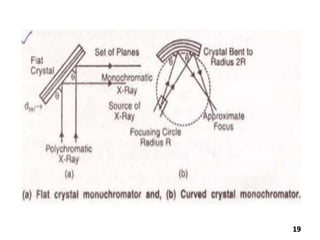
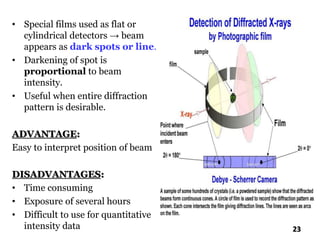
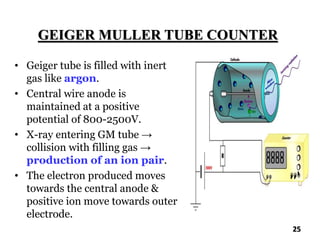
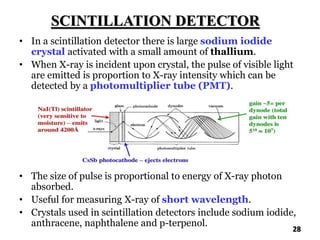
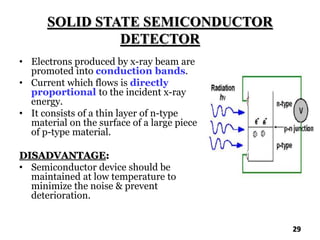
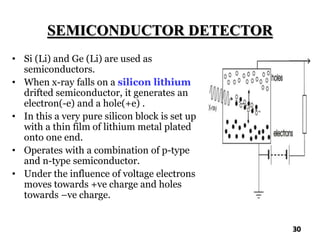
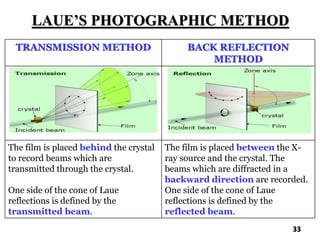
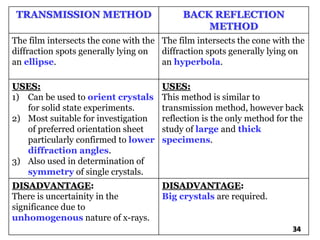
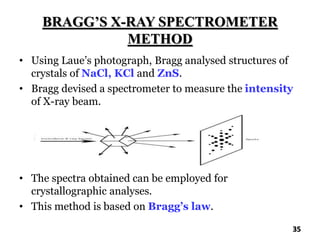
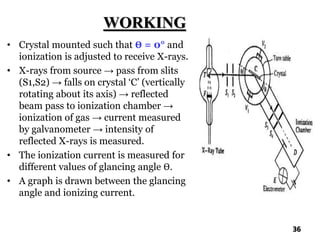
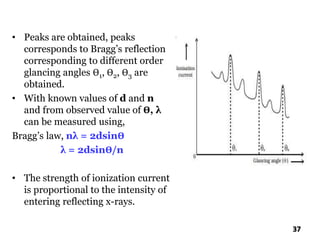
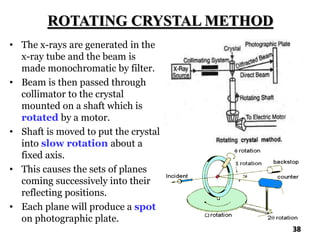
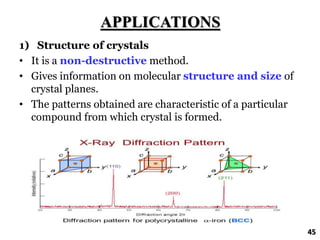
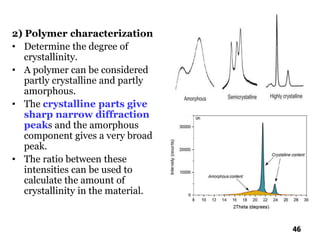
The document discusses various methods of x-ray analysis. It begins by describing how x-rays are produced using a Coolidge tube, which generates x-rays by accelerating electrons into a metal target. It then discusses several x-ray techniques including x-ray diffraction, which is based on constructive interference of x-rays scattered by crystal lattices and is governed by Bragg's law. Finally, it summarizes common methods for x-ray diffraction analysis including transmission methods, back-reflection methods, and Bragg's x-ray spectrometer method which measures diffraction intensities using a rotating crystal.