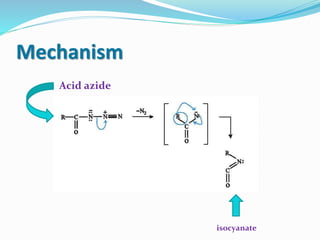
This document summarizes several organic rearrangement reactions: the Cope rearrangement, Claisen rearrangement, and Curtius rearrangement. The Cope rearrangement involves the [3,3]-sigmatropic rearrangement of 1,5-dienes. The Claisen rearrangement is a carbon-carbon bond forming reaction that rearranges allyl vinyl ethers to γ,δ-unsaturated carbonyls. The Curtius rearrangement converts carboxylic acids to isocyanates through an acid azide intermediate. Mechanisms are provided for each reaction.


![Cope rearrangement
The Cope rearrangement is an extensively
studied organic reaction involving the [3,3]-sigmatropic
rearrangement of 1,5-dienes. It was developed by Arthur
C. Cope
For example 3-methyl-1,5-hexadiene heated to 300°C yields
1,5-heptadiene.
The Cope rearrangement causes the fluxional states of the
molecules in the bullvalene family](https://image.slidesharecdn.com/organic-160803052423/85/Rearrangement-4-320.jpg)

![Claisen rearrangement
The Claisen rearrangement (not to be confused with
the Claisen condensation) is a powerful carbon-
carbon bond-forming chemical reaction discovered
by Rainer Ludwig Claisen.
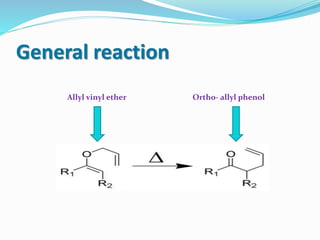
The heating of an allyl vinyl ether will initiate a [3,3]-
sigmatropic rearrangement to give a γ,δ-unsaturated
carbonyl.
Discovered in 1912, the Claisen rearrangement is the first
recorded example of a [3,3]-sigmatropic rearrangement.](https://image.slidesharecdn.com/organic-160803052423/85/Rearrangement-6-320.jpg)