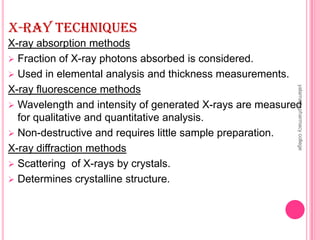
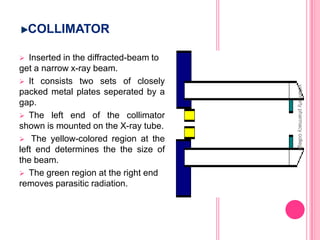
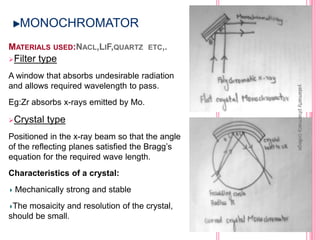
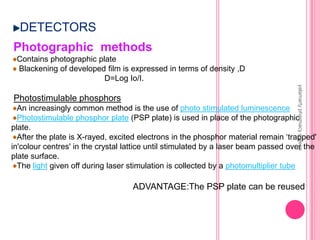
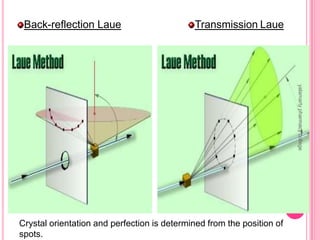
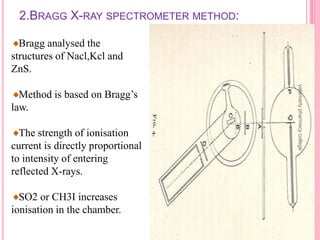
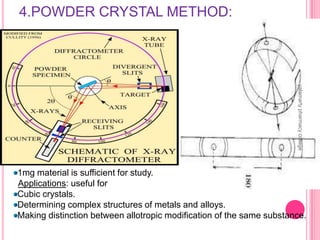
X-Ray Diffraction is a technique used to analyze crystalline structures. It involves using X-rays that are scattered by crystals in a specific pattern determined by Bragg's Law. The document discusses the instrumentation of XRD including the X-ray source, collimator, monochromator, and various detectors. It also covers different XRD methods like Laue, Bragg spectrometer, rotating crystal, and powder crystal methods. Finally, applications of XRD are presented such as determining crystal structures, polymer characterization, and soil classification.



![Characteristic Radiation:
The characteristic lines in an atom's emission spectra are called K, L, M,
... and correspond to the n = 1, 2, 3, ... quantum levels of the electron
energy states, respectively.
α lines (n = 2 to n = 1, or n = 3 to n = 2).
β lines (n = 3 to n = 1 or n = 4 to n = 2).
Moseley found that :1/λ = K2 [Z - σ]2.
Electronic energy levels of an atom
yalamartypharmacycollege](https://image.slidesharecdn.com/x-raydiffraction-130504102406-phpapp02/85/X-ray-diffraction-5-320.jpg)