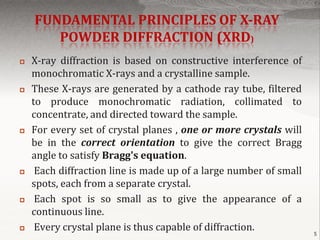
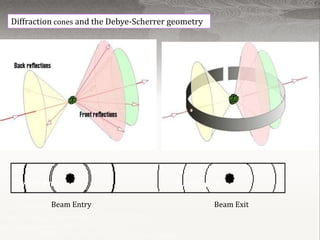
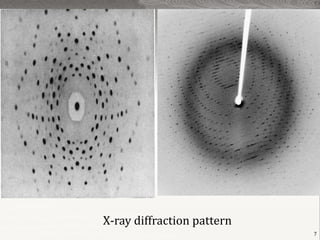
X-ray powder diffraction is a technique used to analyze the crystal structure of materials. Finely powdered samples are bombarded with X-rays, and the resulting diffraction pattern is analyzed. Each material produces a unique pattern that can be used for identification. The instrument works by generating monochromatic X-rays that diffract off the sample, producing concentric cones. The pattern is recorded and analyzed to determine properties like unit cell dimensions. Common applications include phase identification, purity analysis, and structure determination of minerals, compounds, and alloys. The technique is rapid, non-destructive, and requires only small sample amounts.