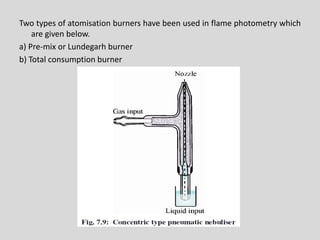
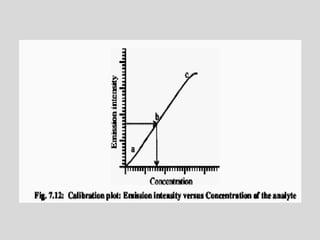
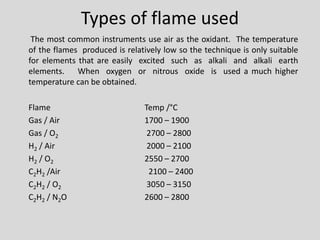
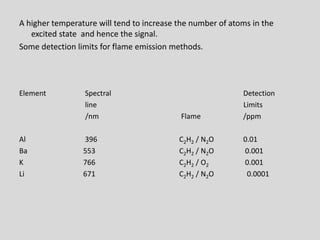
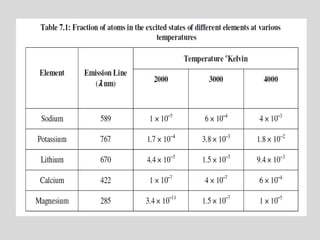
Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms excited in a flame. A sample solution is nebulized into the flame and the atoms are excited to higher energy levels. As they fall back to lower levels, they emit light of specific wavelengths. A monochromator isolates the desired wavelength which is measured by a detector. The intensity of light emitted relates to the concentration of the element in the original sample solution, allowing for quantitative analysis. Flame photometry can be used to detect various metals at concentrations as low as parts per million.