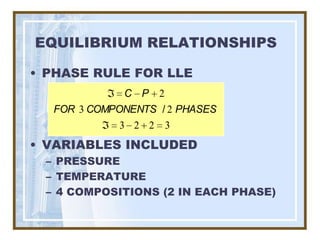
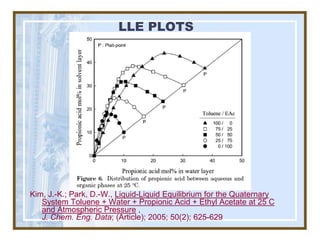
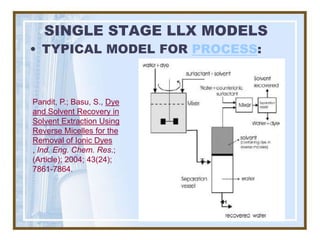
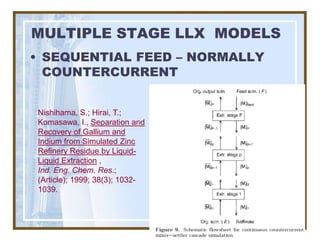
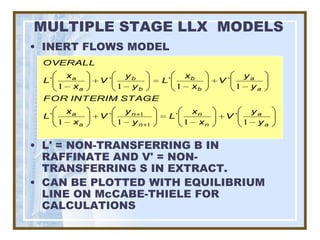
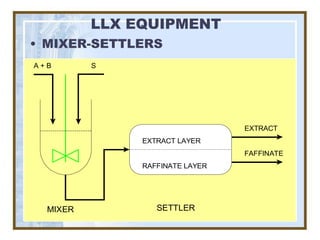
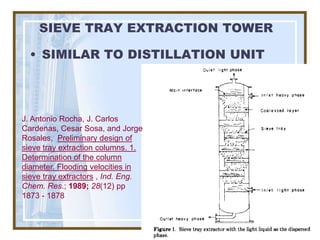
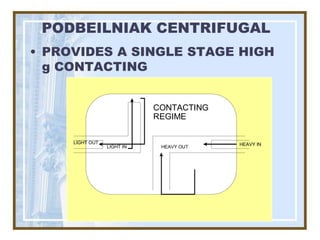
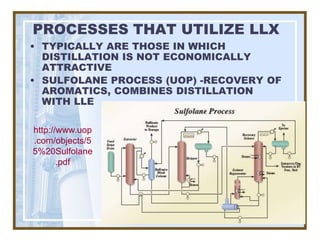
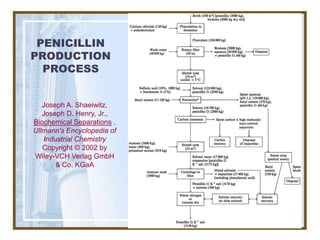
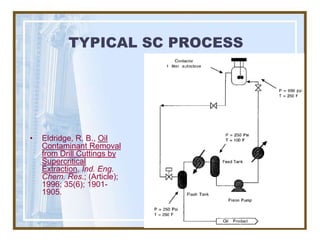
This document discusses liquid-liquid extraction (LLE) models, applications, equilibrium relationships, and equipment. Key points include: LLE can be modeled using phase rules and mass balances accounting for temperature, pressure, and compositions of both phases; equilibrium data is needed to calculate stage-by-stage extraction; common equipment includes mixer-settlers, spray columns, and sieve tray towers; and processes that utilize LLE include separation of aromatics and biopharmaceutical production.