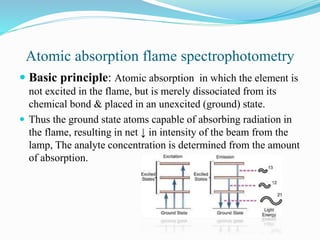
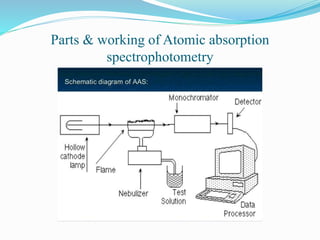
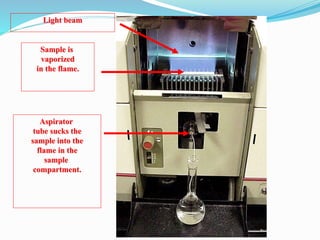
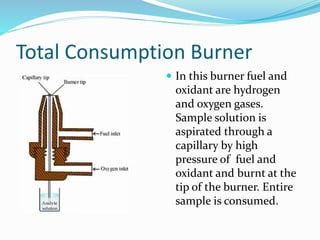
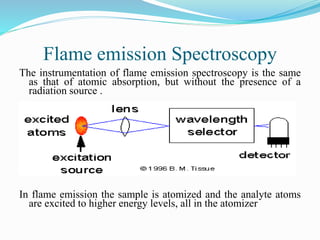
This document discusses atomic absorption spectrophotometry and flame emission spectrophotometry. It begins by explaining the basic principles of atomic absorption spectrophotometry, which measures the concentration of elements by detecting the absorption of light by atoms. It then describes the parts and working of atomic absorption spectrophotometry instruments. Next, it covers flame emission spectrophotometry, which measures the radiation emitted by excited atoms to determine concentrations. The document concludes by comparing the two techniques and discussing some sources of interference.