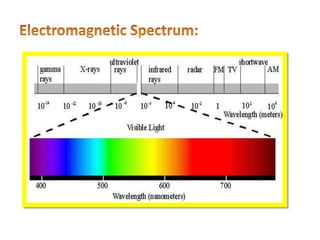
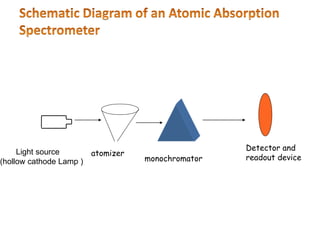
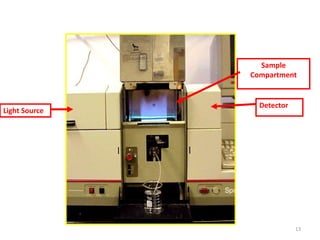
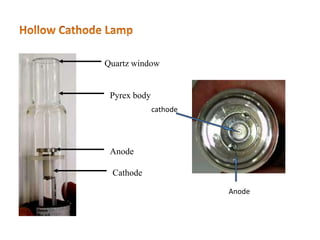
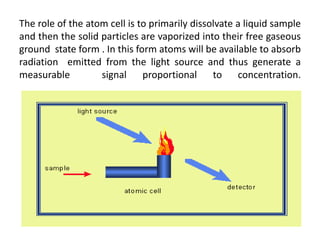
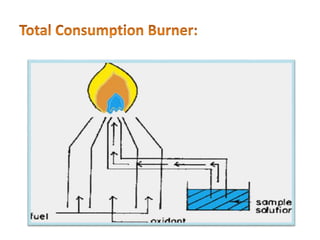
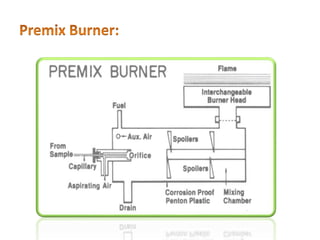
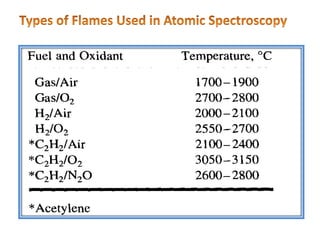
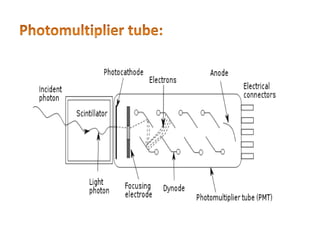
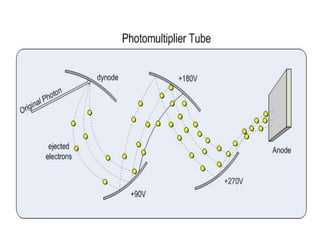
This document discusses atomic absorption spectroscopy (AAS). AAS involves using a light source to emit electromagnetic radiation that matches an element's characteristic wavelength. When the element's atoms absorb this radiation in their gaseous ground state, the intensity of the radiation decreases. By measuring this decrease, AAS can quantify the concentration of metals and some non-metals in liquid samples. The key components of an AAS instrument are the light source, atomizer, monochromator, and detector. The atomizer converts the sample into free atoms, while the monochromator selects the characteristic wavelength and the detector measures the absorbed intensity.