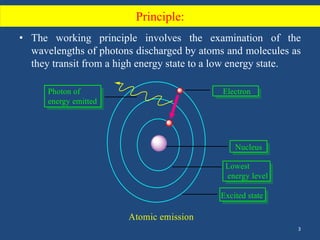
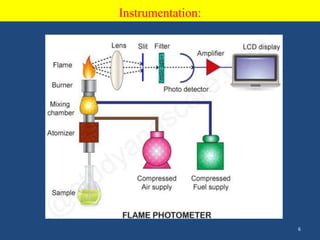
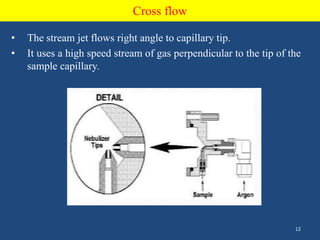
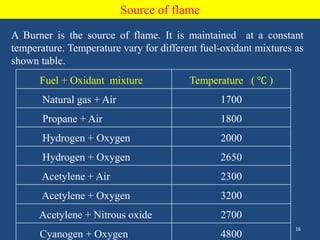
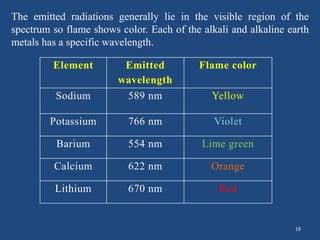
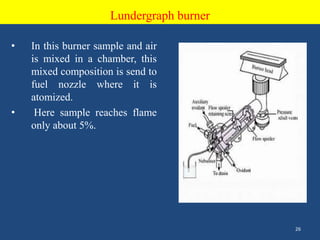
Atomic emission spectroscopy uses quantitative measurement of optical emission from excited atoms to determine analyte composition. The sample is nebulized and introduced into an excitation source like a flame where atoms are raised to excited states. Upon returning to lower states, atoms emit radiation of characteristic wavelengths, which are isolated and measured with a photodetector. The intensity of light emitted is proportional to the concentration of the emitting element in the sample.