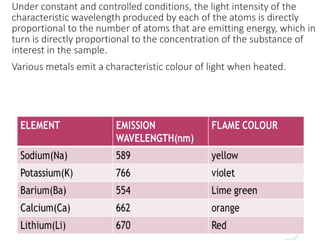
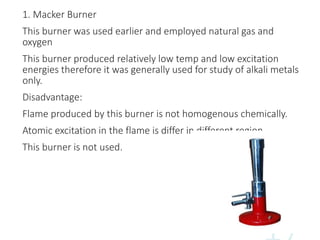
This document discusses flame photometry, which uses the intensity of light emitted from heated metal atoms to determine the concentration of certain metal ions. It describes the basic working principles, including how a metal salt solution is atomized in a flame, causing the atoms to emit light of specific wavelengths. It then summarizes the key components of a flame photometer instrument, including the sample delivery system, fuel/oxidant sources, monochromator for separating wavelengths, and detector for measuring light intensities. The document provides details on common flame types and considerations for efficient atomization and excitation of metal samples for analysis.