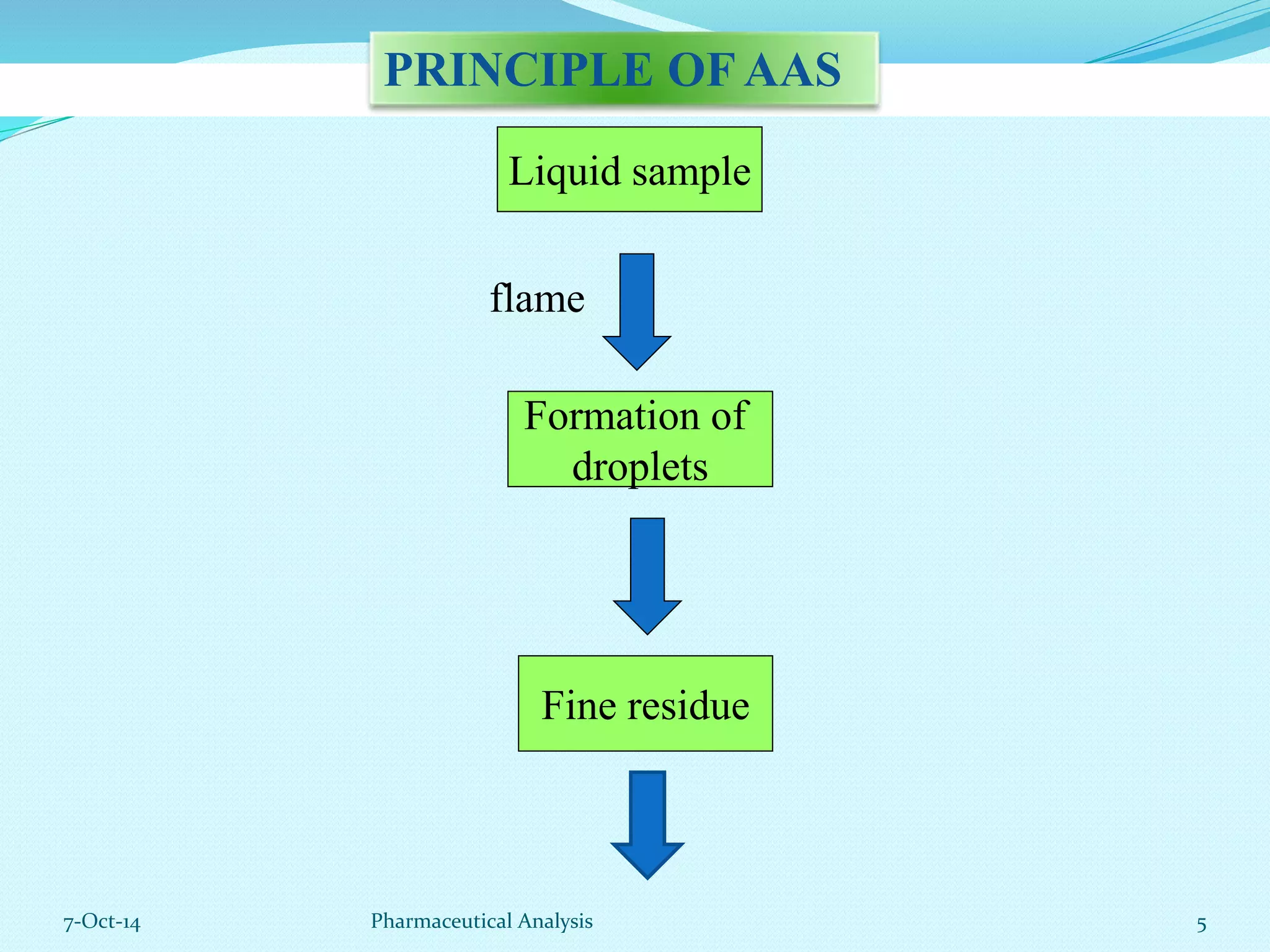
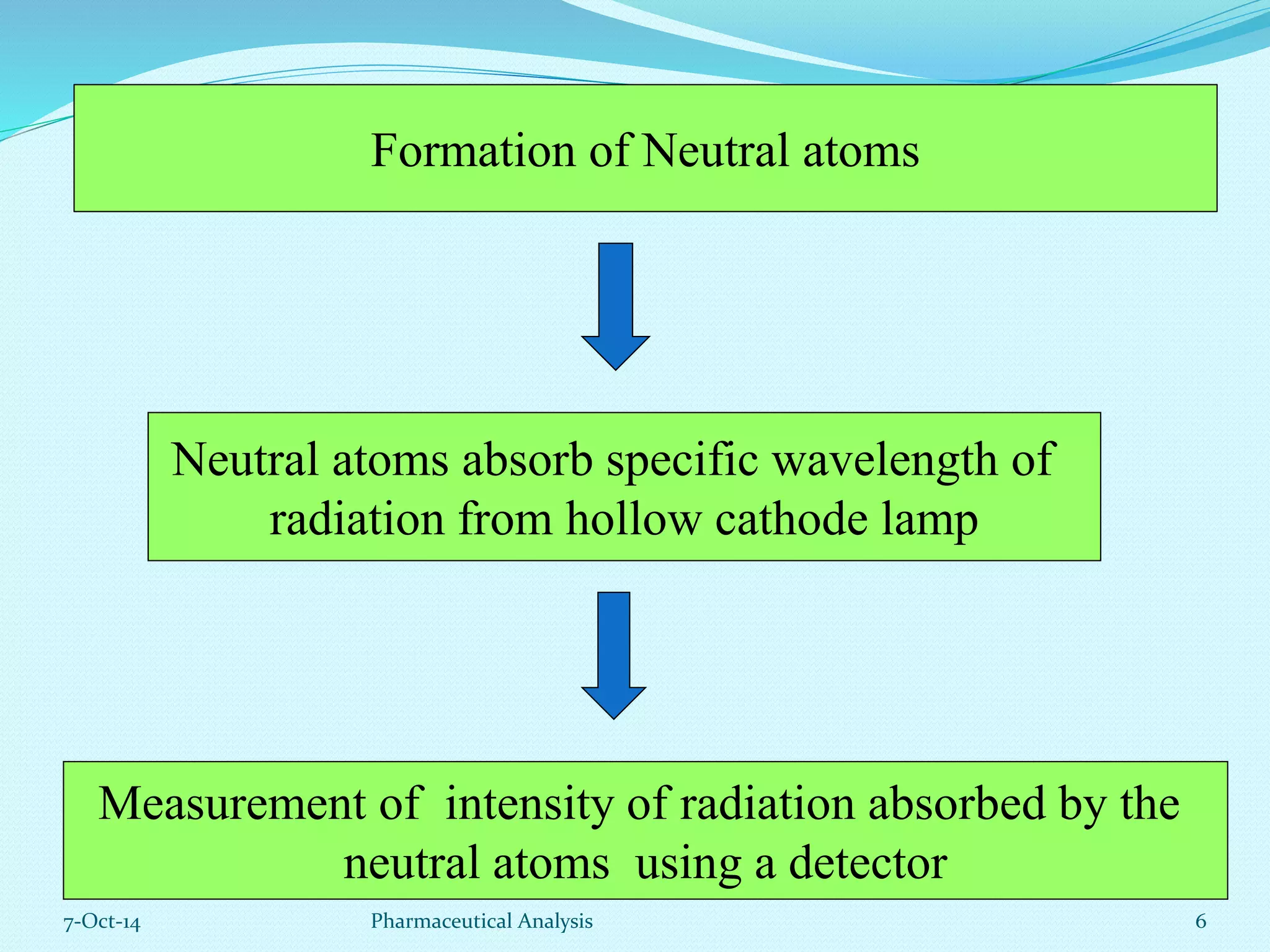
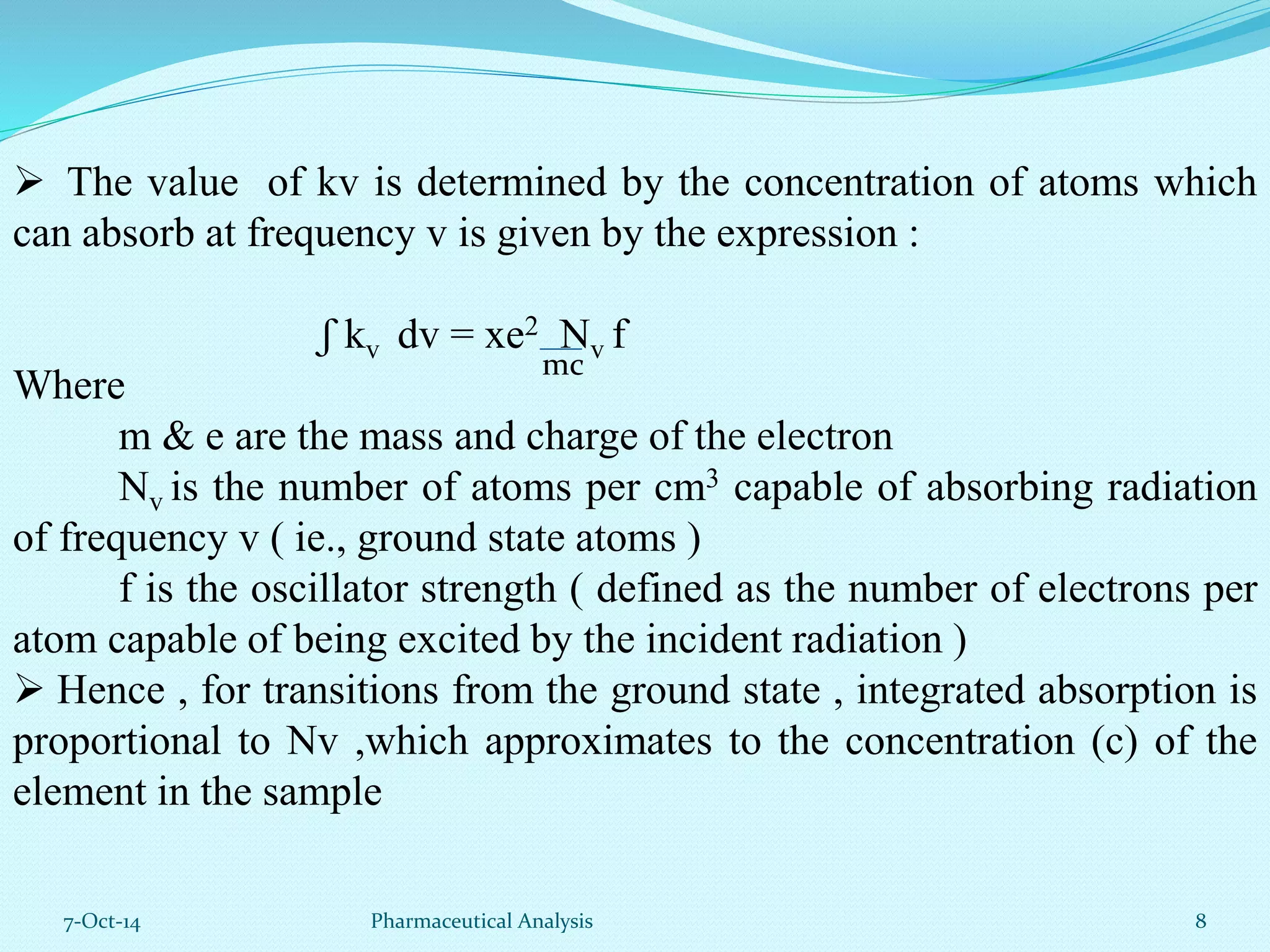
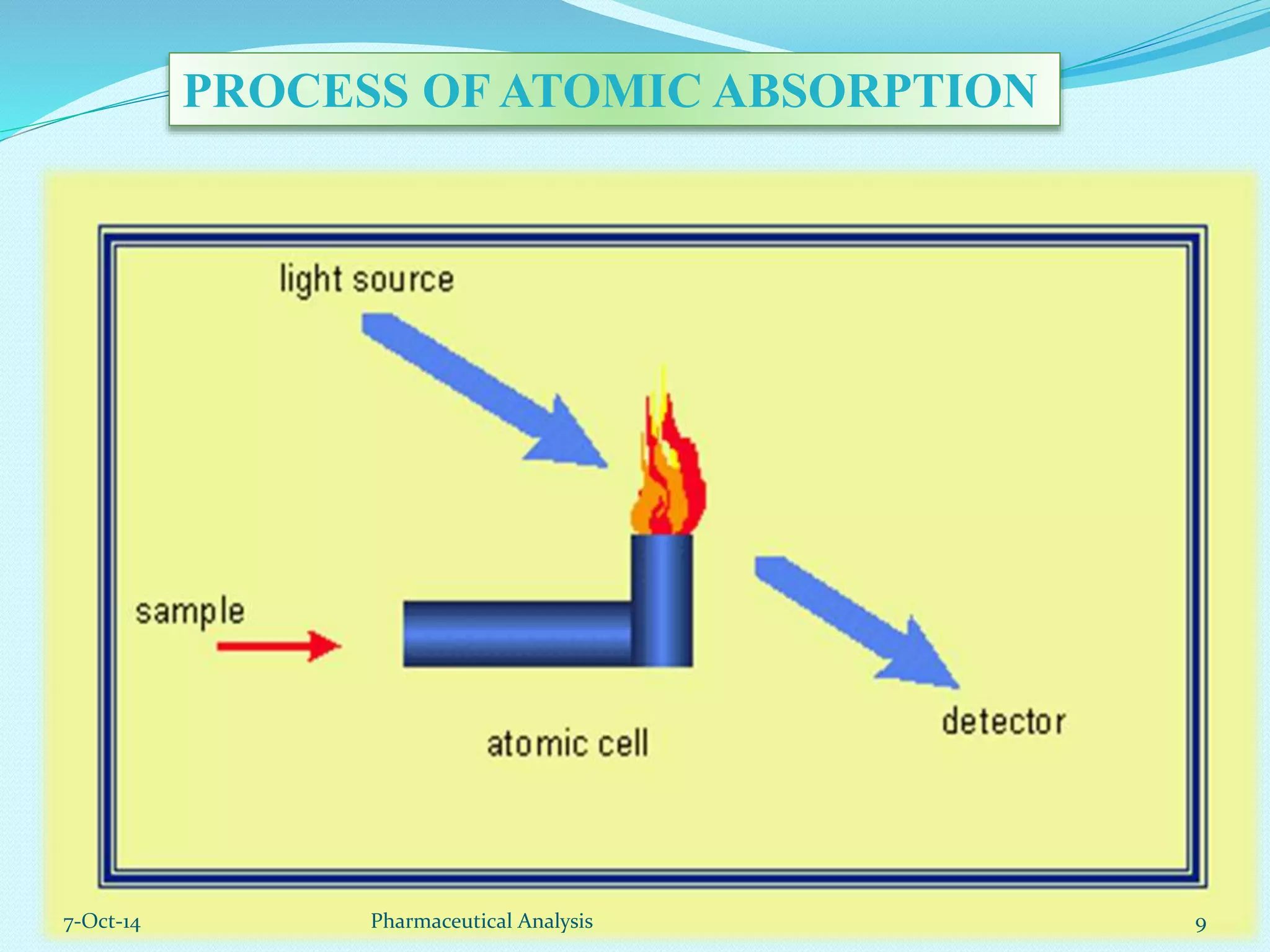
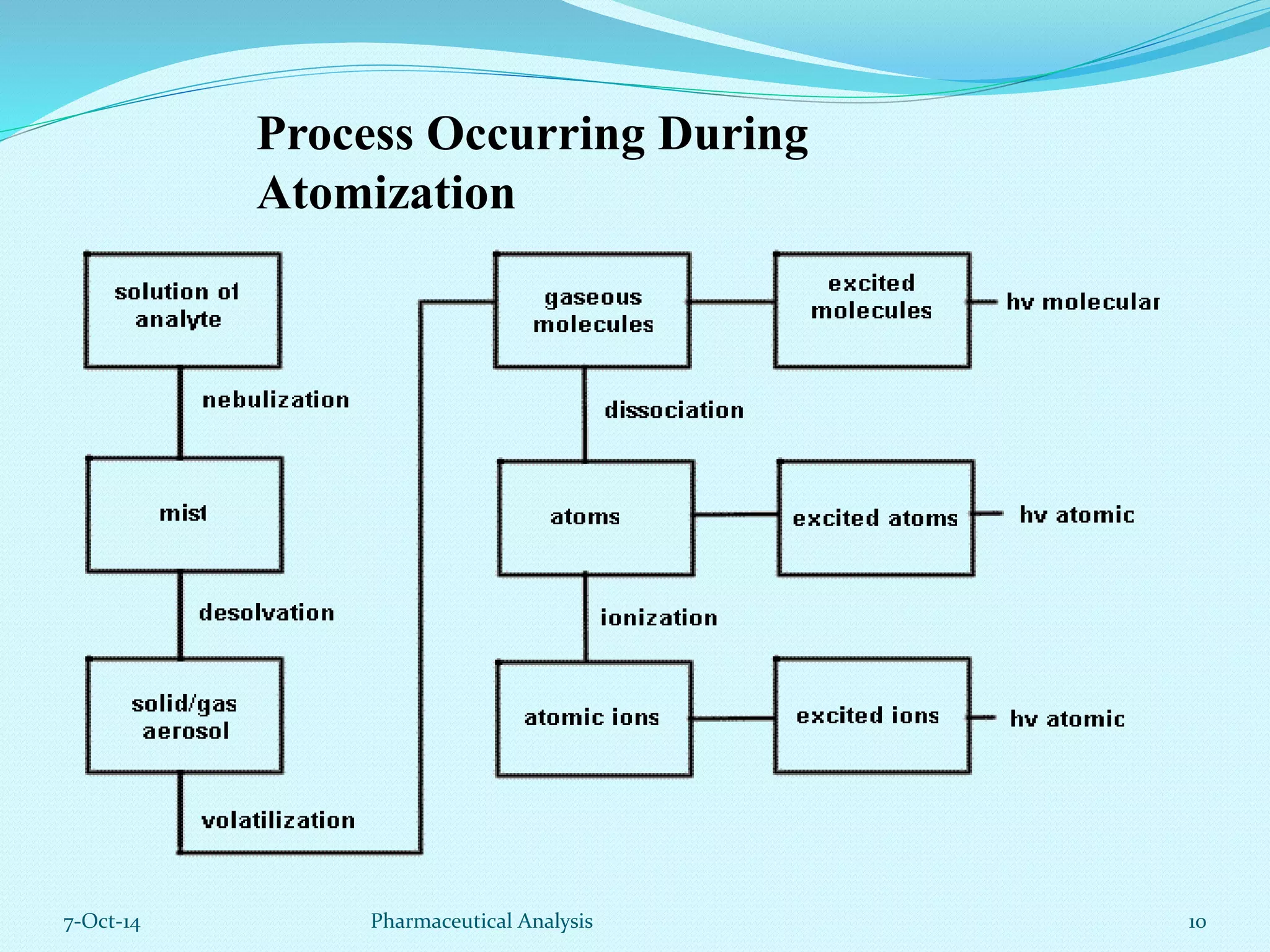
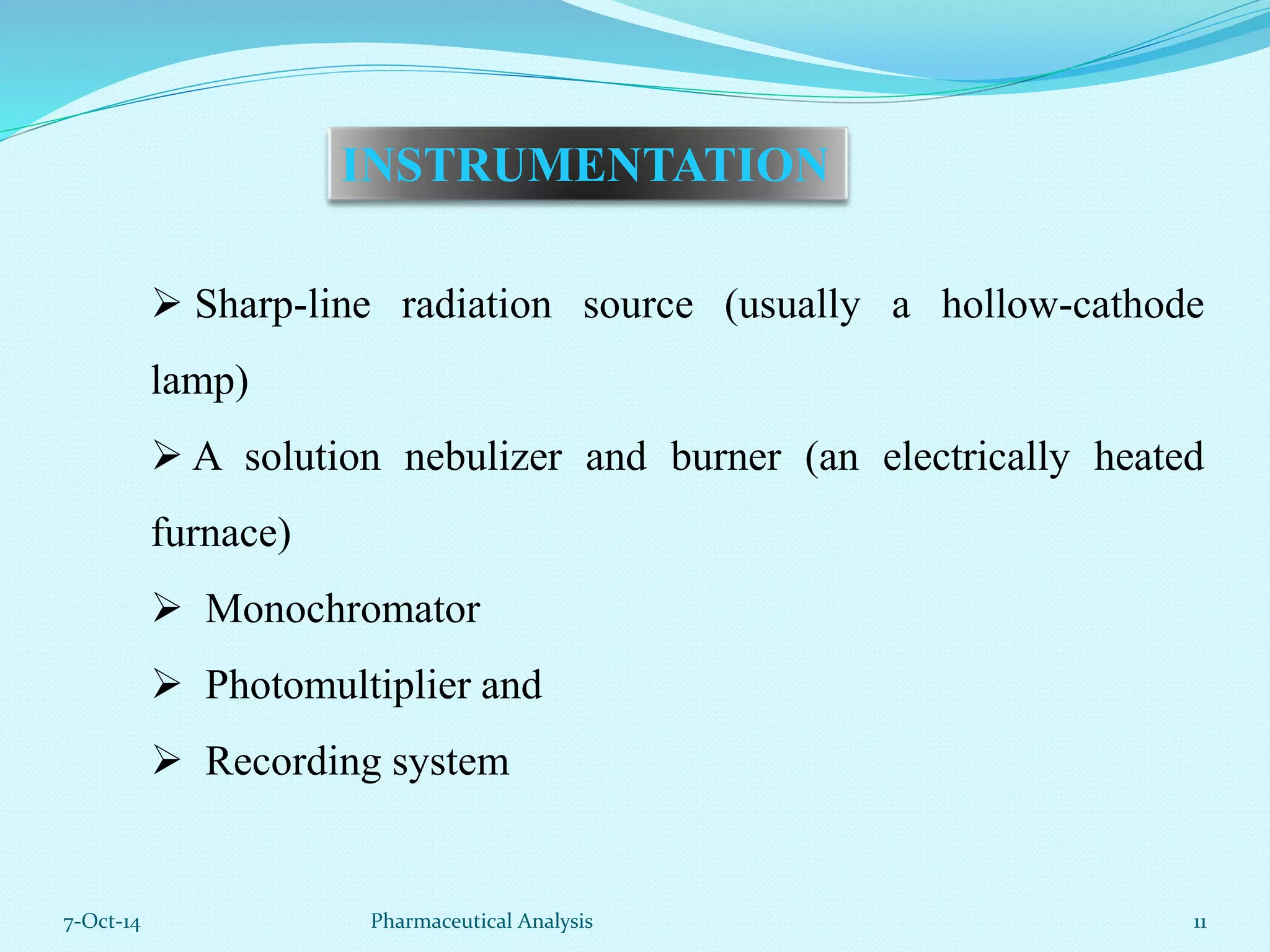
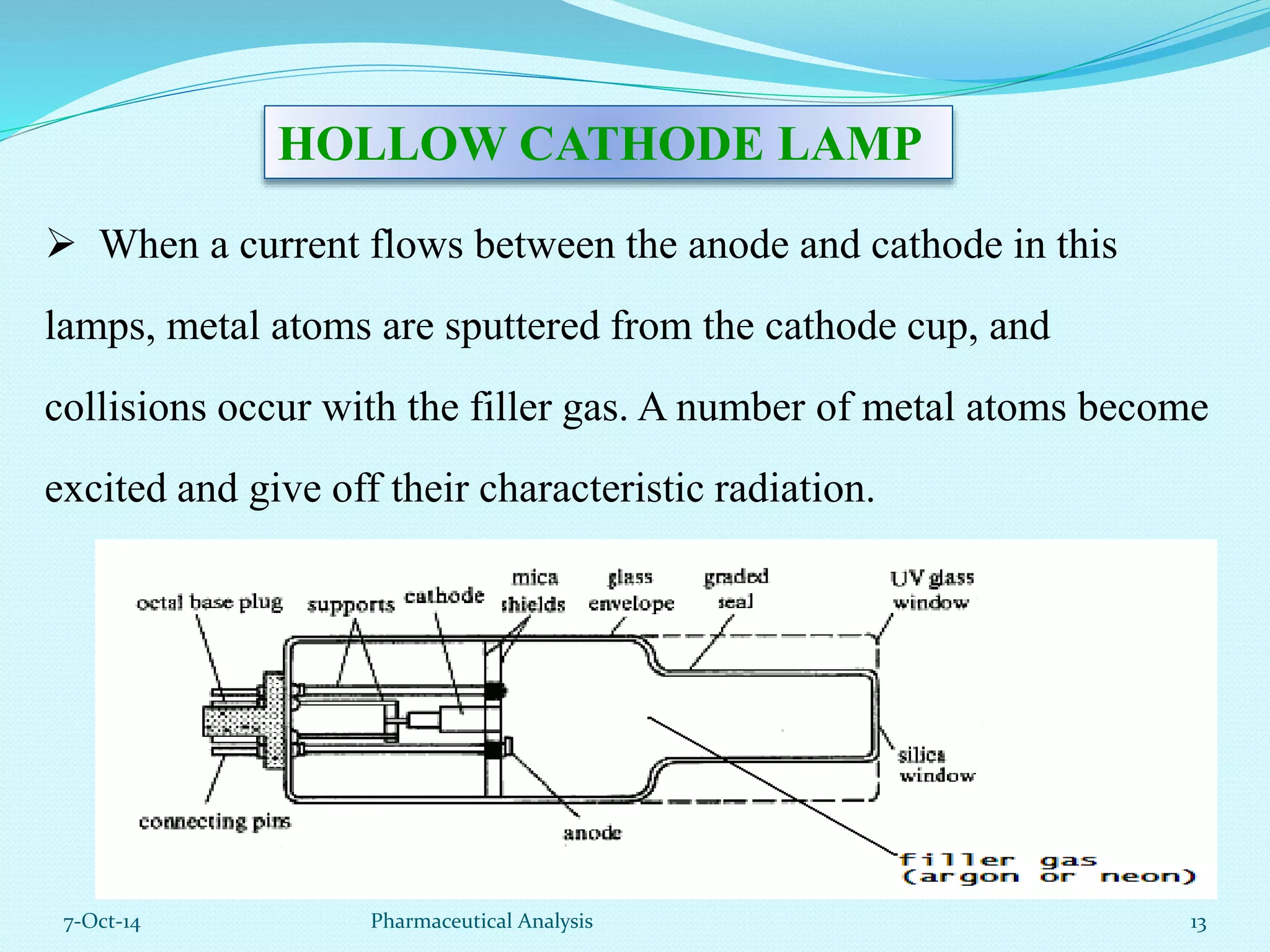
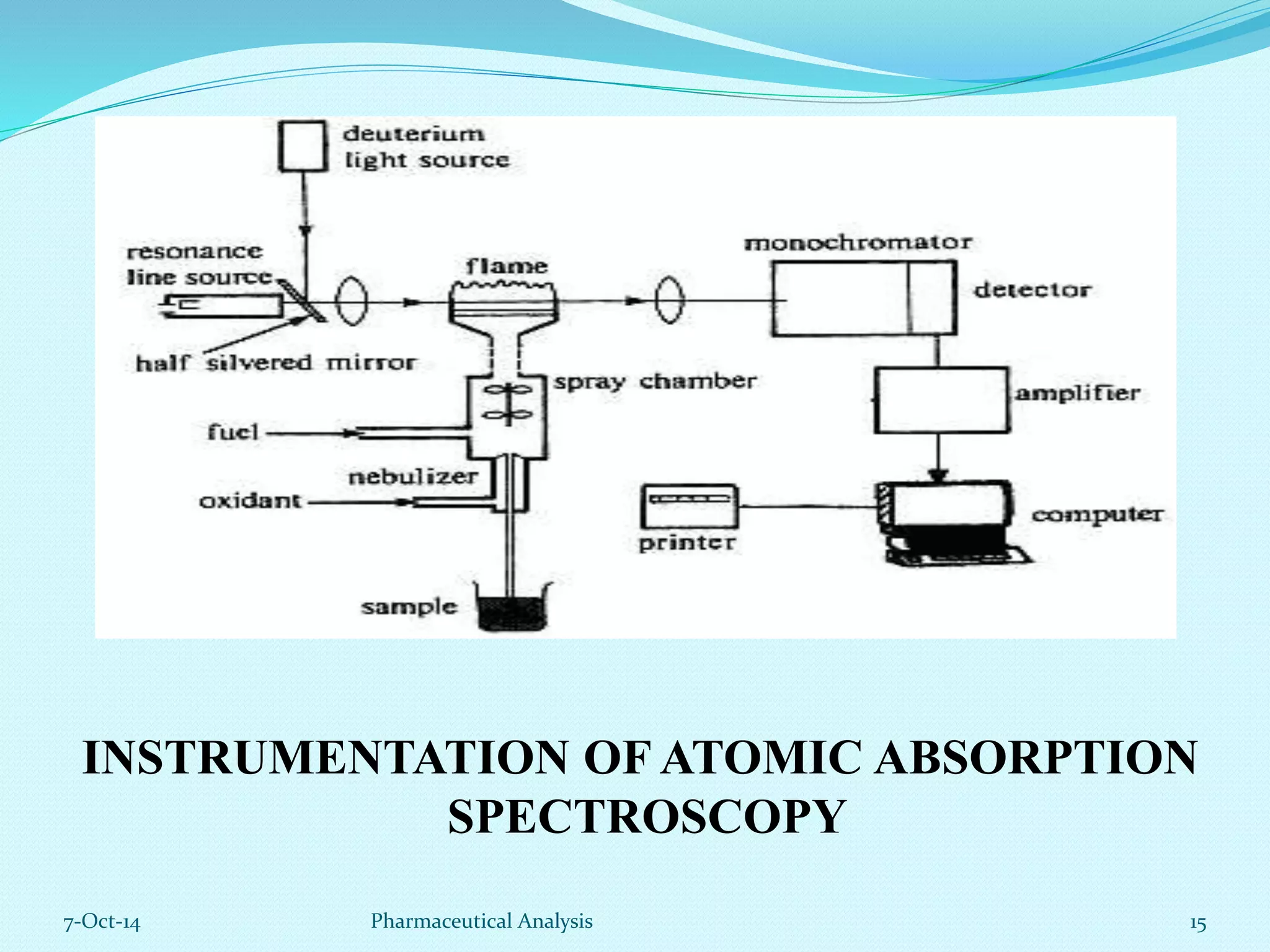
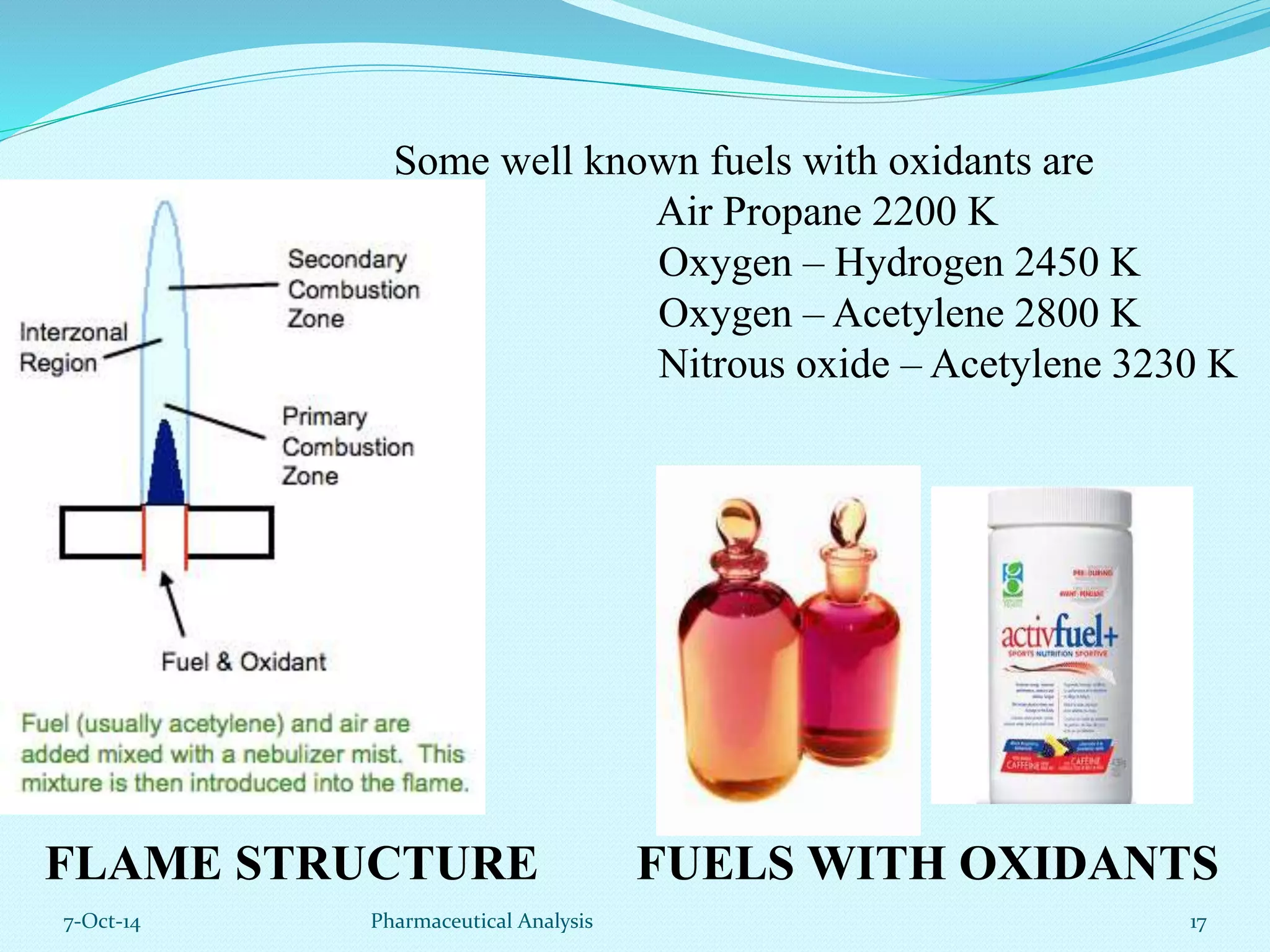
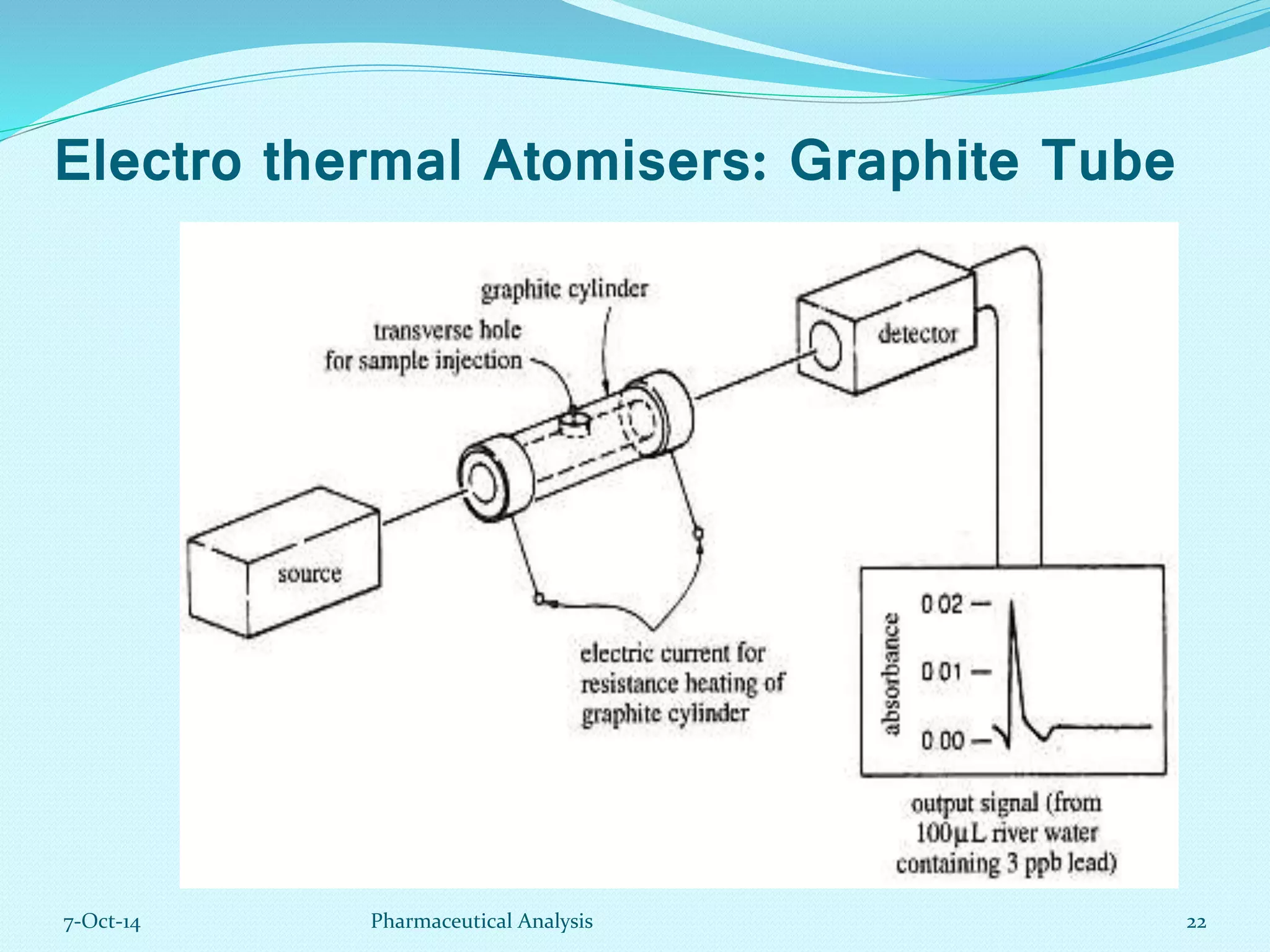
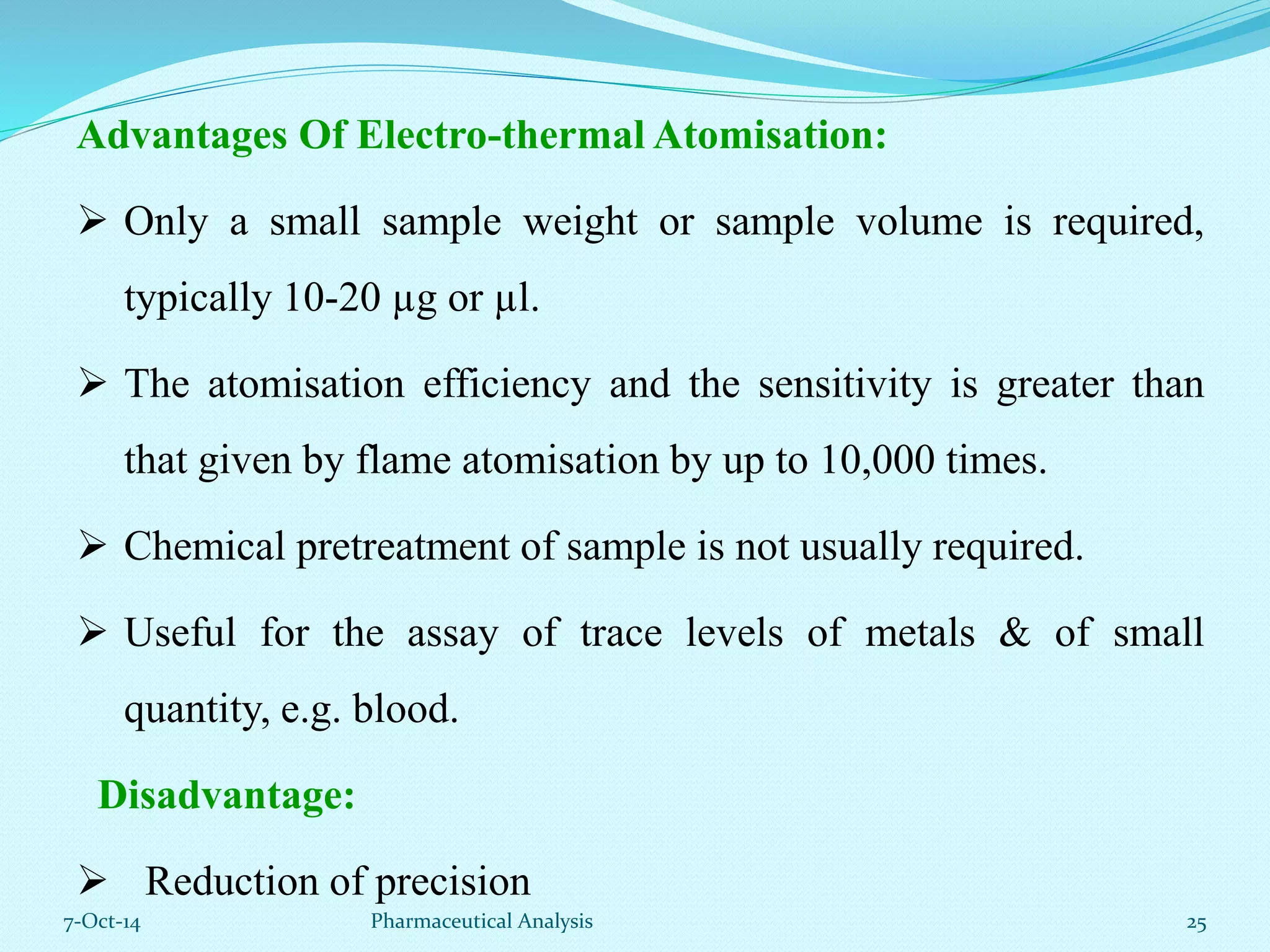
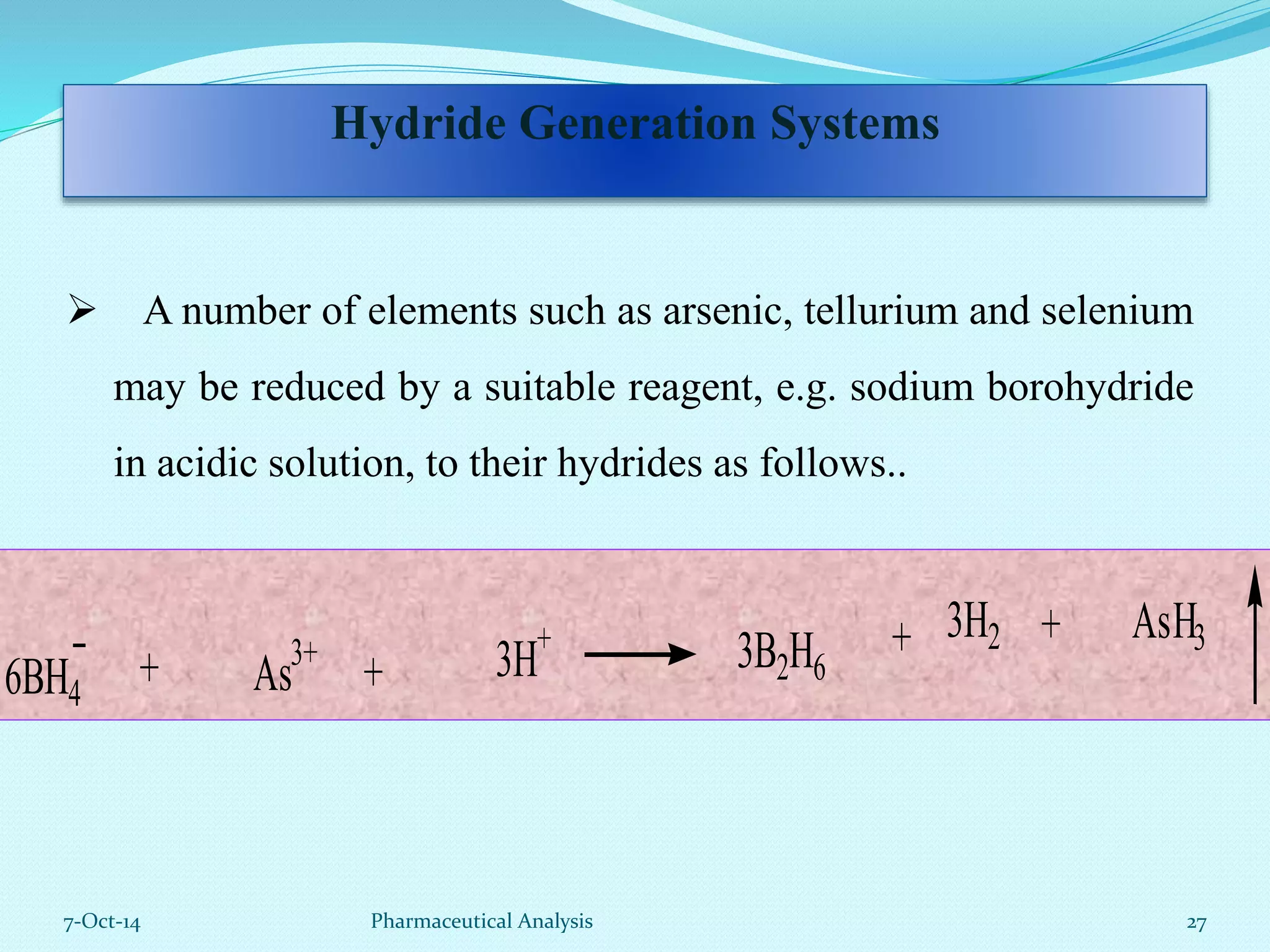
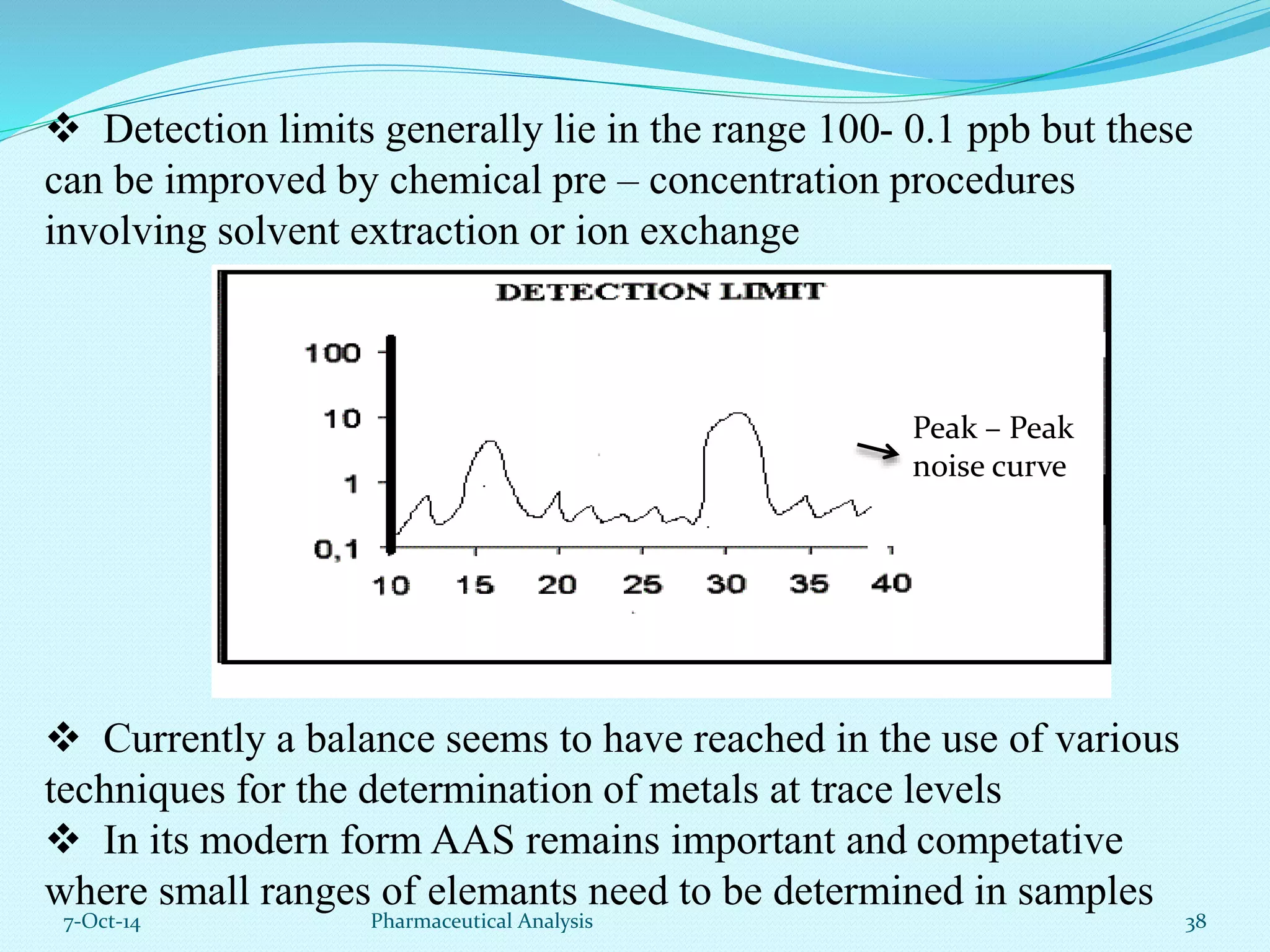
The document presents an overview of atomic absorption spectrophotometry (AAS), detailing its introduction, principles, instrumentation, and techniques for analyzing metals at trace levels. AAS utilizes sharp-line radiation sources to measure the absorption of specific wavelengths of radiation by neutral atoms in the vapor state, and discusses advantages, disadvantages, and potential applications across various fields, including pharmaceutical analysis. Key references and acknowledgments are also included to support the presented content.