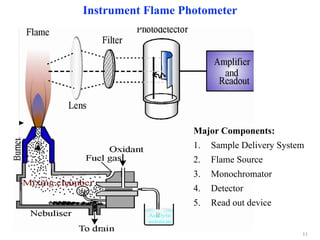
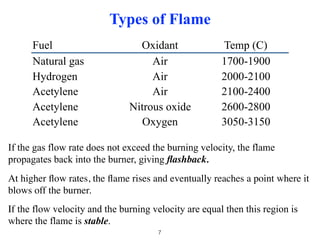
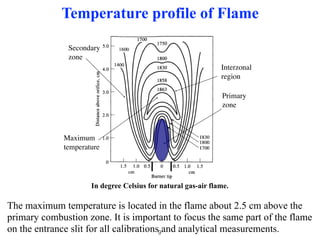
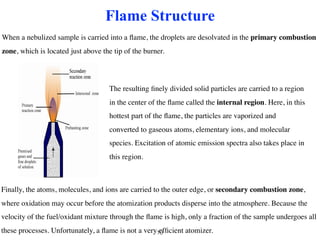
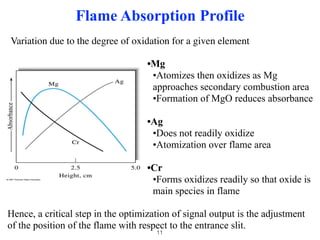
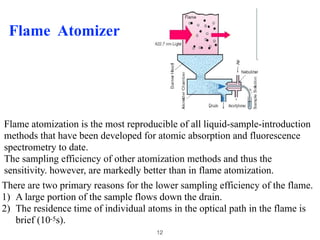
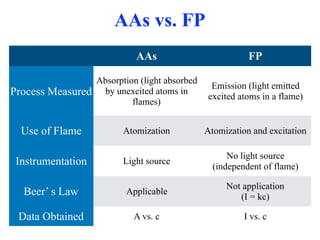
Flame photometry is a technique that uses the characteristic colors emitted from flames to determine the presence of certain metal ions. When metal salts are aspirated into a flame, the metals are excited and emit light at characteristic wavelengths. The intensity of the emitted light is proportional to the concentration of the metal ion in solution. Common metal ions that can be analyzed using flame photometry include sodium, potassium, lithium, and calcium.