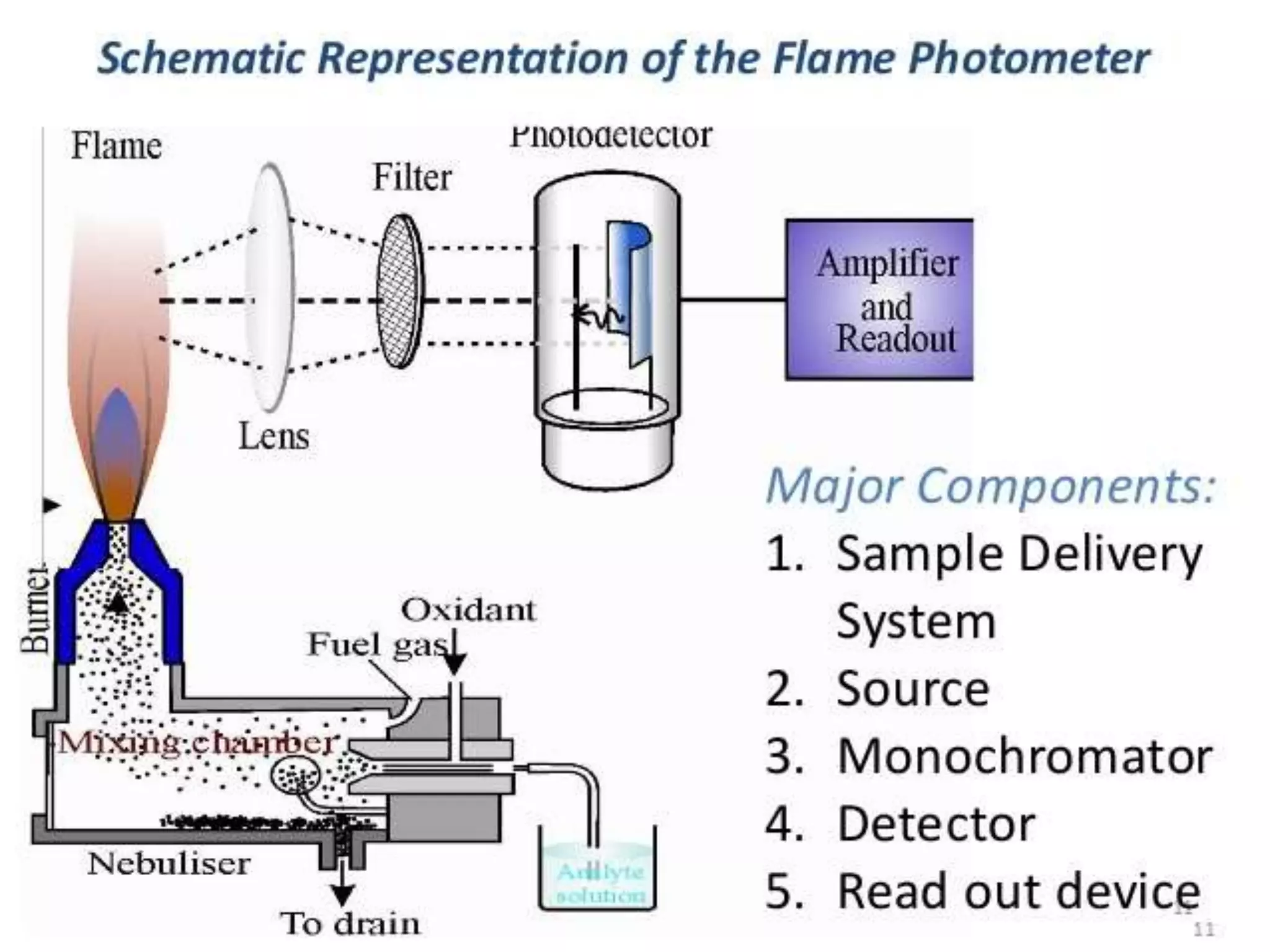
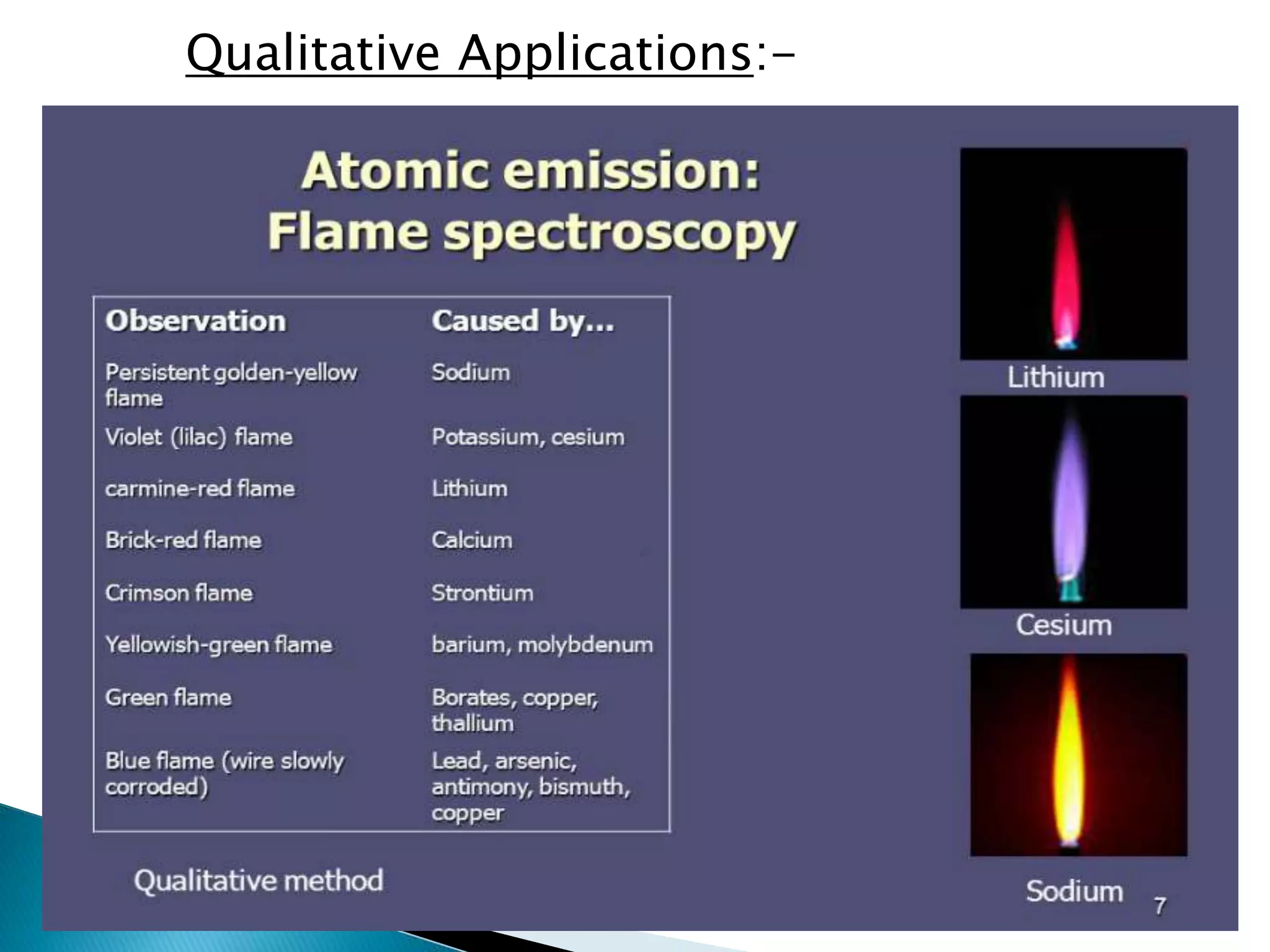
Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Atoms are excited by thermal collisions in the flame and emit radiation of characteristic wavelengths when returning to lower energy states. A monochromator selects the desired wavelength which is measured by a detector. This technique is used to qualitatively and quantitatively analyze samples for alkali and alkaline earth metal content. It provides a simple, fast analysis but has limitations such as spectral interferences and inability to detect non-metals.