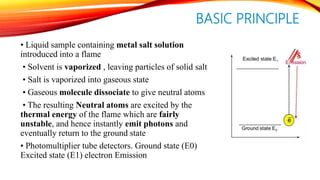
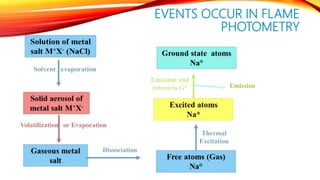
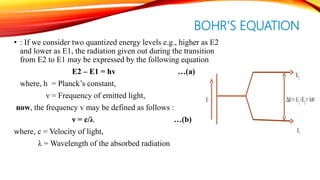
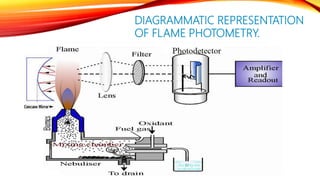
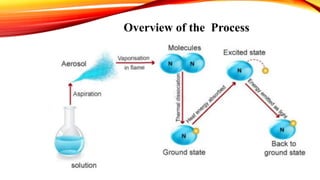
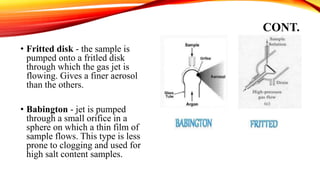
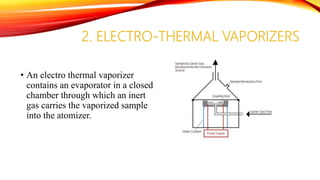
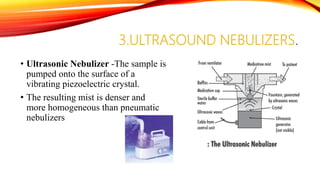
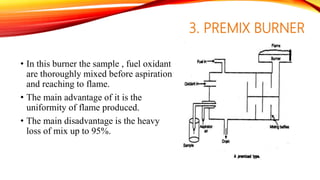
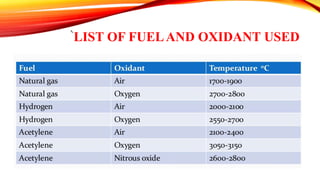
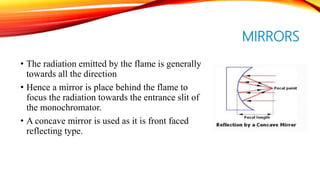
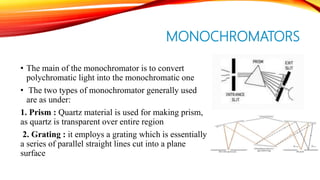
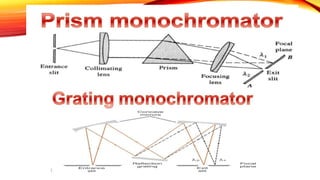
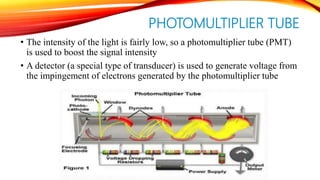
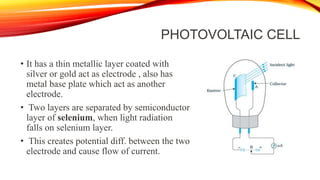
Flame photometry is a technique that uses the intensity of light emitted from an element in a flame to determine its concentration. Samples are nebulized and introduced into a flame where atoms are excited and emit light of characteristic wavelengths. A monochromator separates this light, which is detected and the intensity measured to quantify the elemental concentration. Sodium, potassium, lithium, and calcium are commonly analyzed using this technique in applications like serum analysis and soil testing.