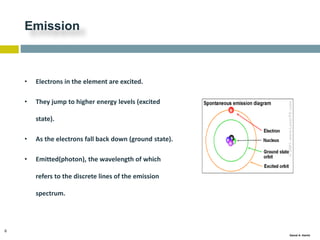
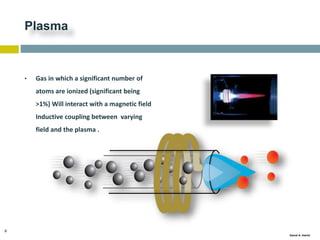
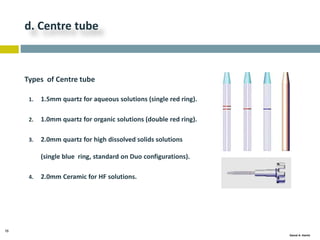
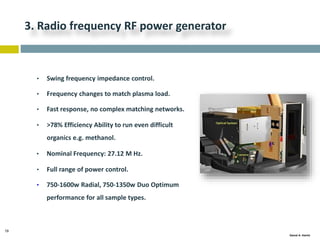
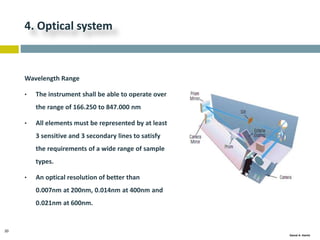
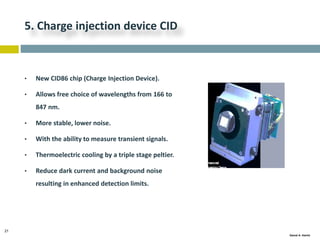
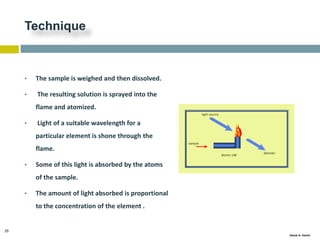
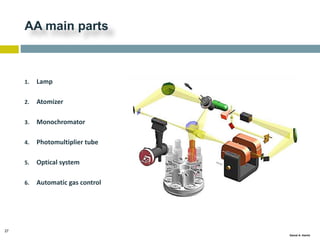
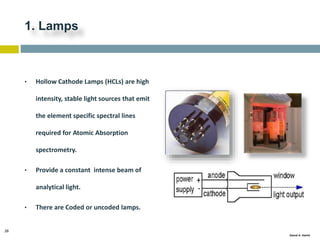
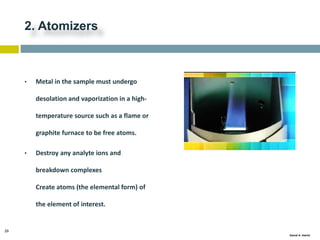
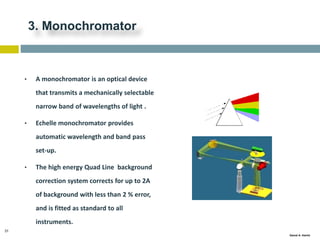
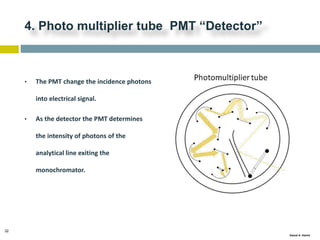
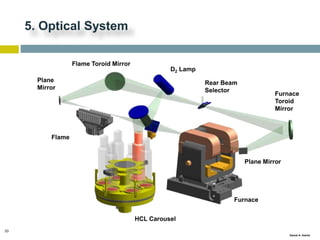
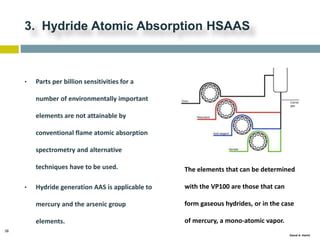
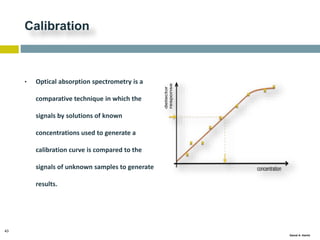
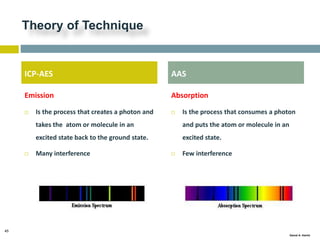
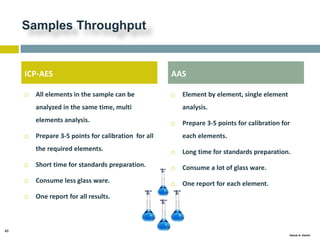
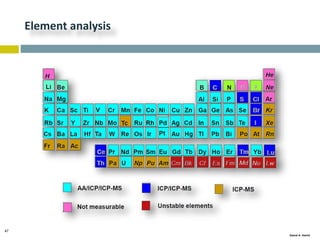
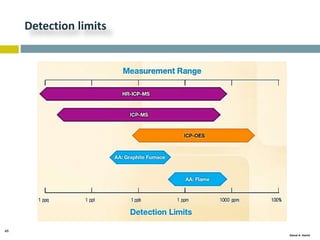
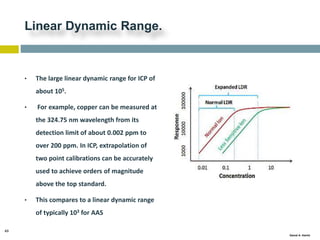
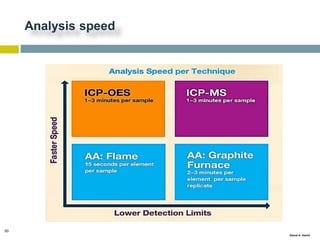
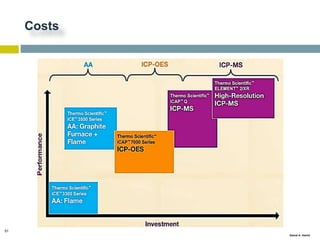
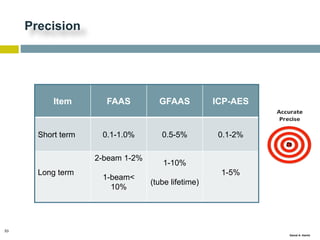
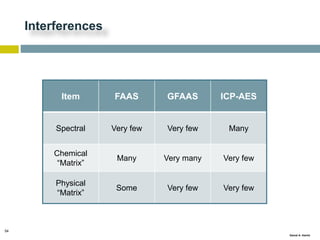
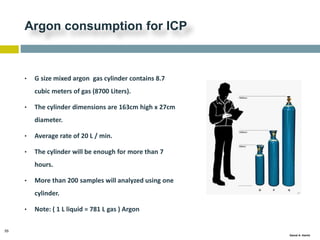
This document discusses and compares inductively coupled plasma atomic emission spectroscopy (ICP-AES) and atomic absorption spectroscopy (AAS). It outlines the fundamental parts and techniques of each method including sample introduction, atomization, and detection. Key differences are described such as ICP-AES being able to analyze multiple elements simultaneously while AAS is single-element. The document also compares factors such as detection limits, linear ranges, analysis speeds, costs, ease of use, interferences, and applications.