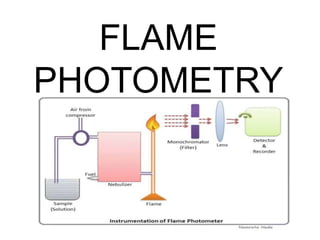
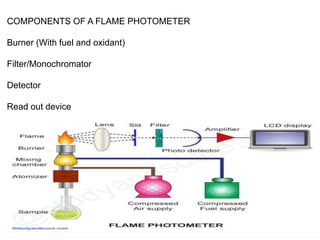
Flame photometry uses the principle that metal ions emit light of characteristic wavelengths when excited by a flame. The intensity of light emitted is proportional to the concentration of metal ions. In flame photometry, the sample solution is nebulized and atomized in a flame, causing the metal ions to emit light. A filter selects the characteristic wavelength, which is detected and its intensity measured, allowing determination of the metal ion concentration. Flame photometry is used to analyze samples for concentrations of ions such as sodium, potassium, lithium, and calcium.