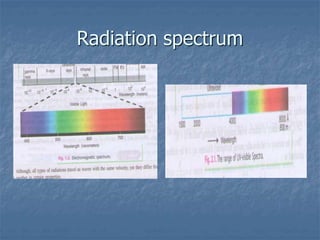
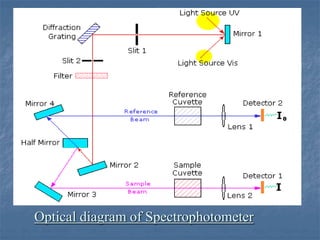
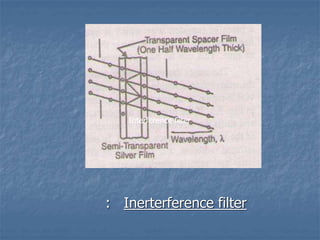
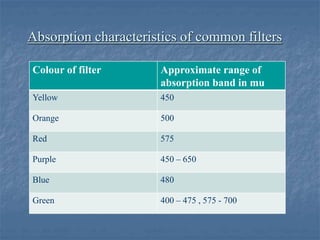
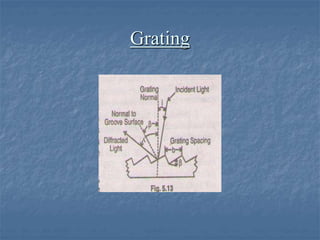
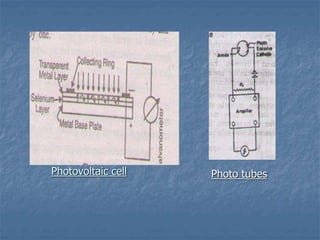
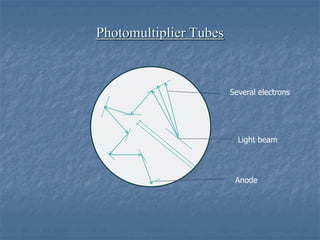
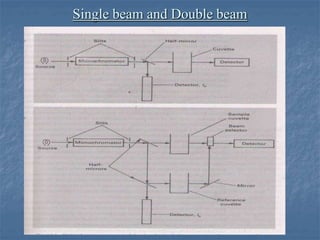
The document provides an overview of UV-visible spectrophotometry, detailing its principles, laws of absorption, and instrumentation components. It discusses Lambert's and Beer's laws, highlighting their application in determining substance concentrations and absorbance. The document also describes different types of spectrophotometers and their uses in analytical chemistry, including qualitative and quantitative analysis.