Atomic absorption spectrometry analyzes samples by breaking them down into atoms and measuring the absorption of light of a specific wavelength by those atoms. It works by vaporizing a sample in a flame and passing light from a hollow cathode lamp of the element being analyzed through the flame. The extent to which light is absorbed is proportional to the number of ground state atoms present and can be used to determine the elemental composition of a sample. Key components of an AAS instrument include the hollow cathode lamp light source, the flame atomizer and fuel system to vaporize samples, a monochromator to select the wavelength of light, and a photomultiplier detector to measure light absorption.





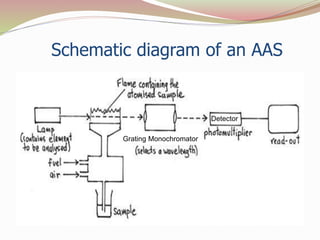


![Instrumentation – Fuel & Atomizer
The sample is prepared in aqueous solution.
A flame is created, usually using ethyne & oxygen (fuel) & the
solution is subjected into the flame by nebulizer.
The flame gases flowing into the burner create a suction that
pulls the liquid into the small tube from the sample container.
This liquid is transferred to the flame where the sample is
atomized [mixing the sample with air to create fine droplets].
The metal atoms then absorb light from the source (cathode
lamp). 9](https://image.slidesharecdn.com/atomicabsorptionspectroscopy-151012135527-lva1-app6891/85/Atomic-absorption-spectroscopy-9-320.jpg)