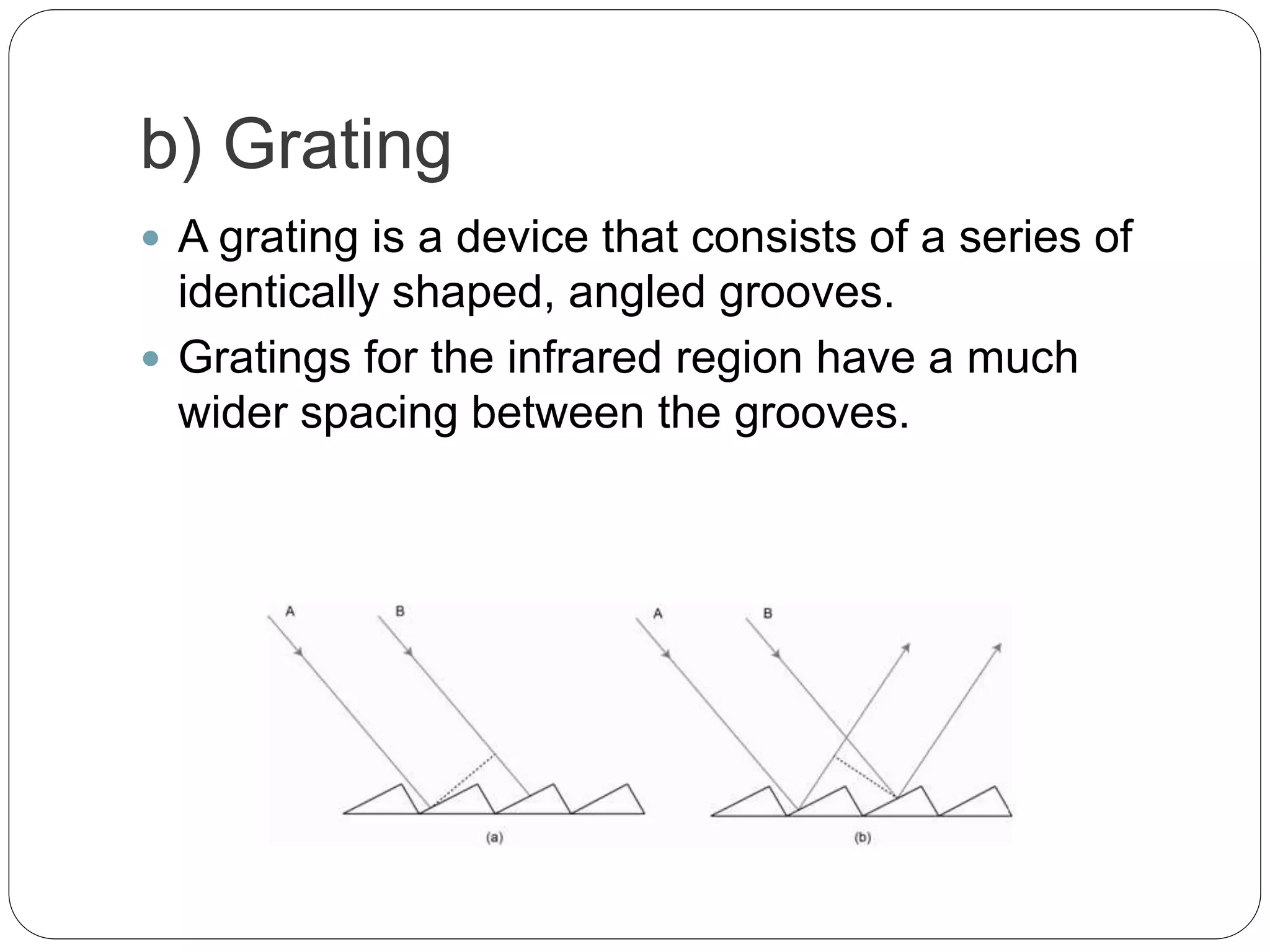
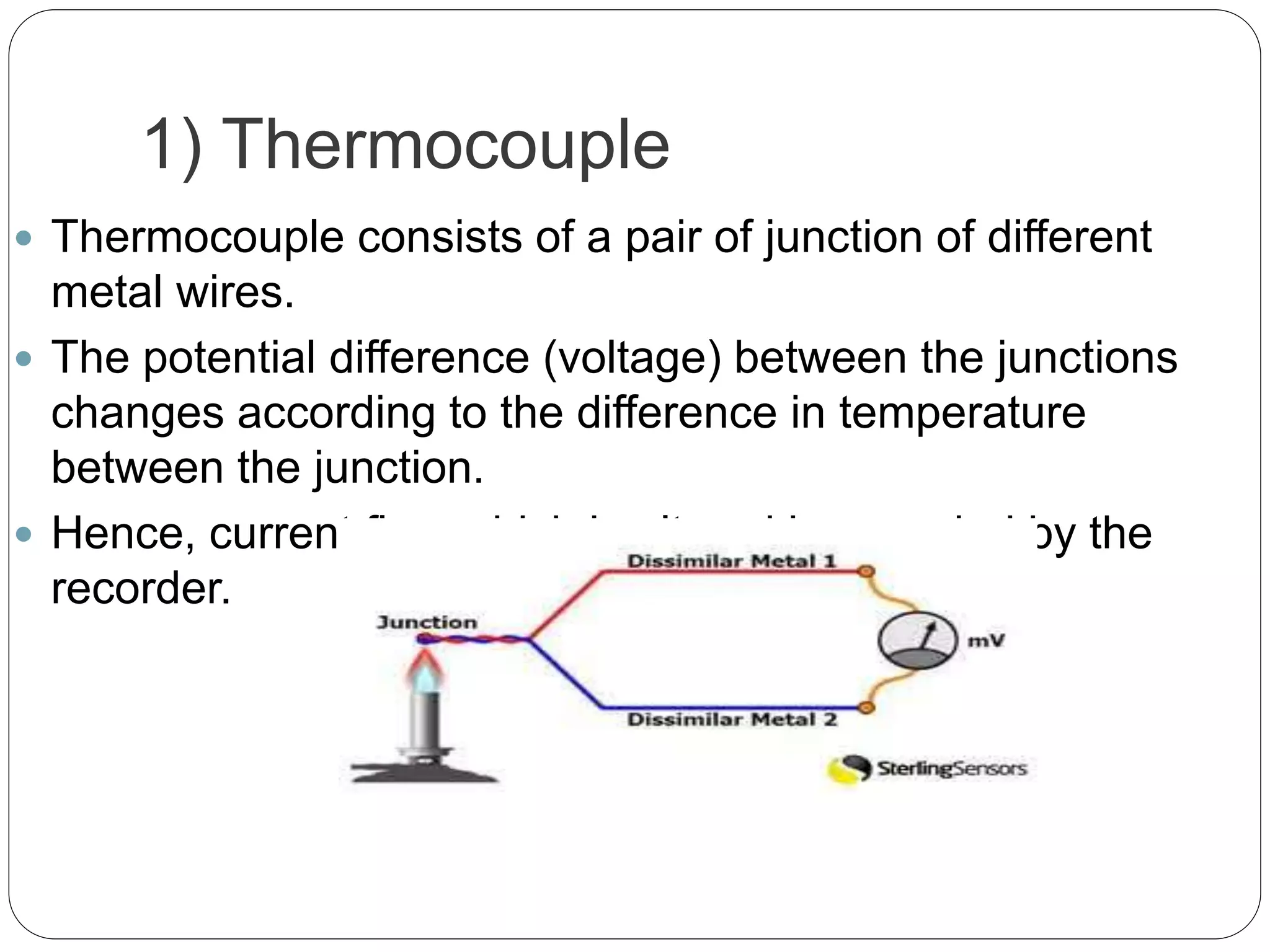
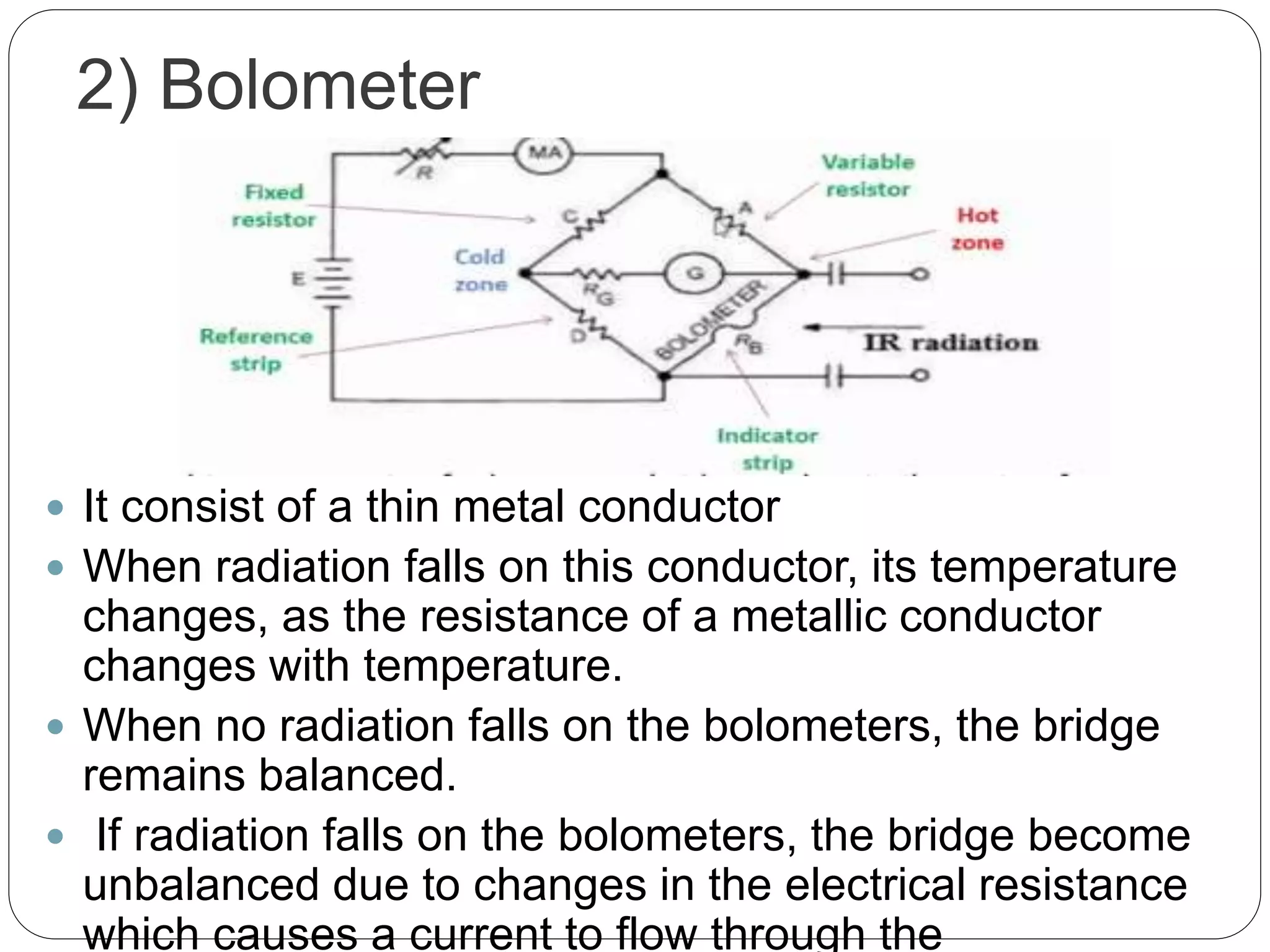
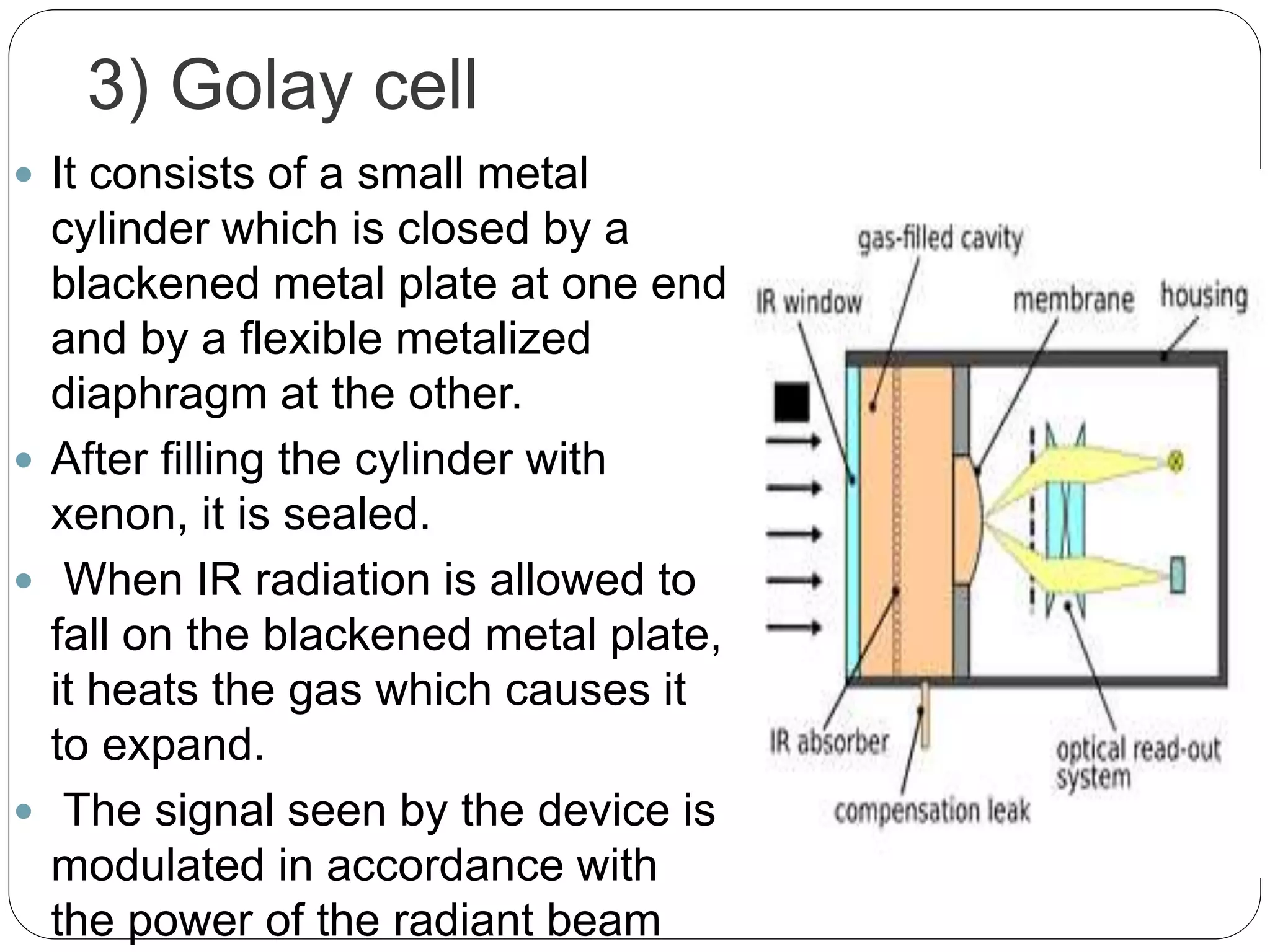
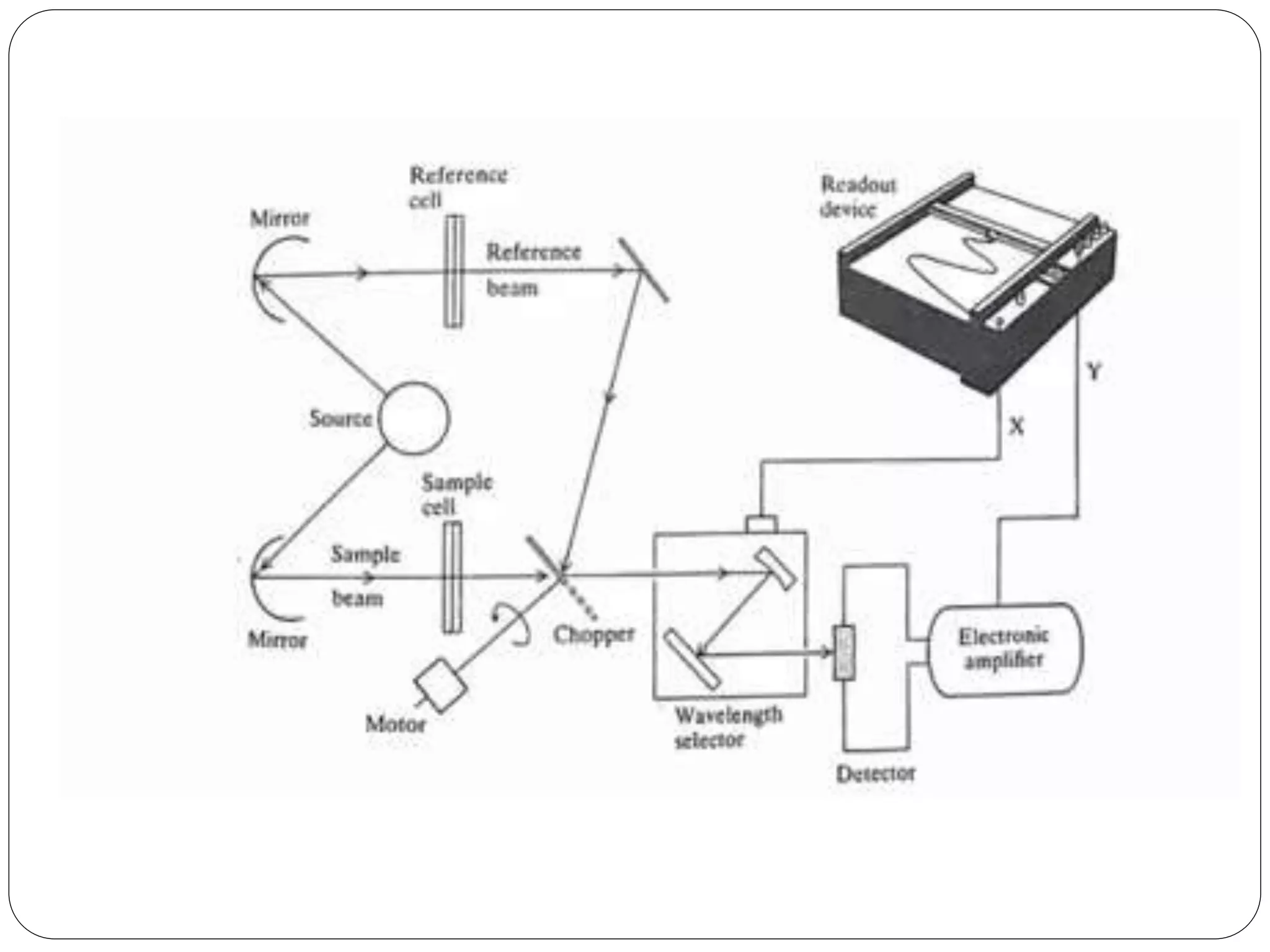
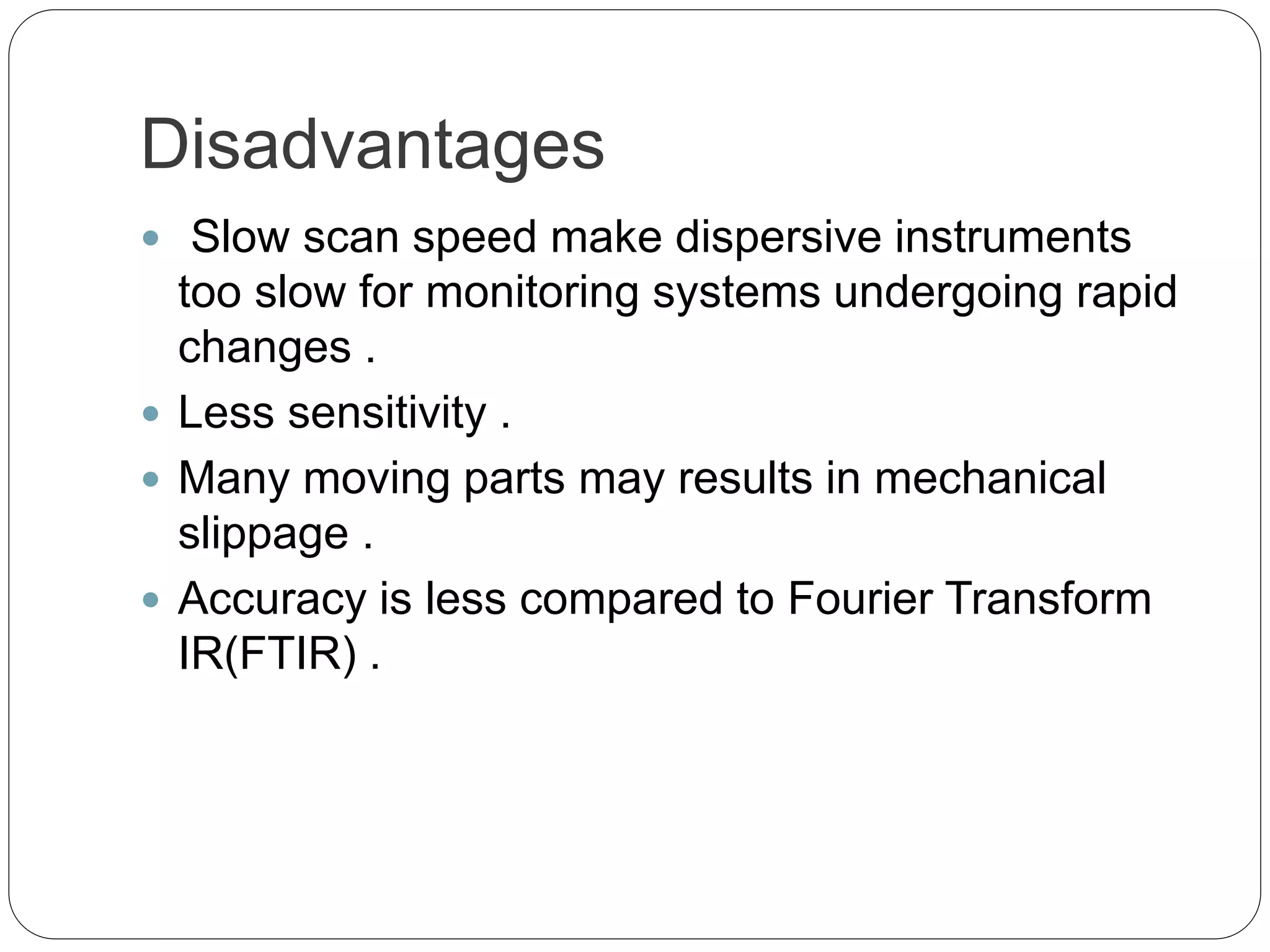
This document discusses the principles, instrumentation, and applications of a dispersive infrared spectrophotometer. It describes how this type of IR spectrometer works by using radiation sources like globars or Nernst glowers, monochromators to separate wavelengths, and detectors like photo detectors or thermal detectors to analyze the absorbed infrared wavelengths. Key applications of dispersive IR spectrophotometers include identifying organic and inorganic compounds by their functional groups and determining molecular structure and orientation. However, it also notes some disadvantages like slower scan speeds and less sensitivity compared to Fourier transform IR spectrometers.