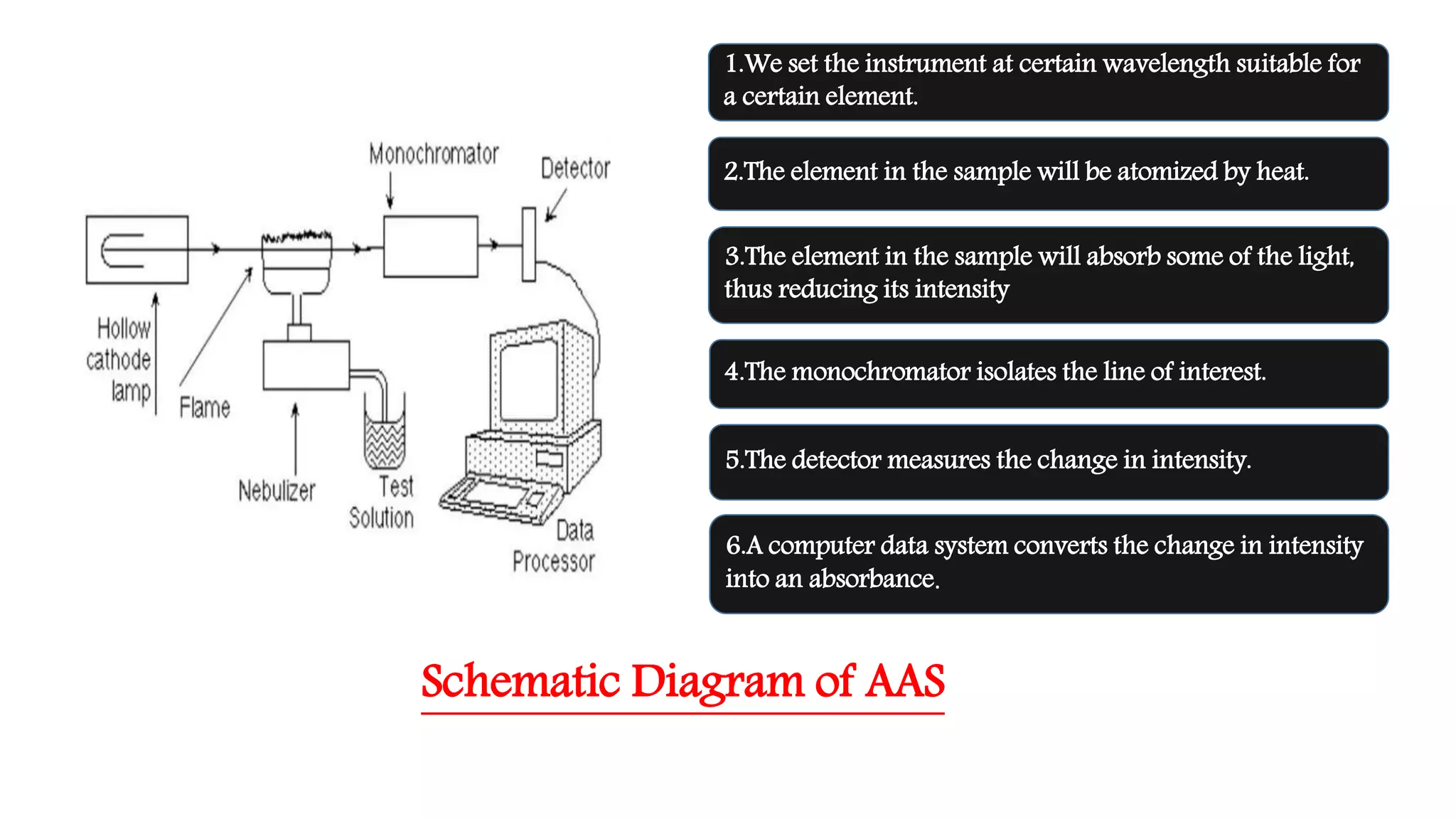
Atomic absorption spectroscopy is a technique used to determine the concentration of chemical elements in solution or solid samples. It works by vaporizing the sample into free atoms that can absorb light at specific wavelengths. The amount of light absorbed is measured and used to determine the concentration of elements in the sample. It has been used since the 1950s and can analyze over 70 elements. It provides a simple and reliable way to quantify metals in applications like environmental monitoring, food testing, and pharmaceutical analysis.