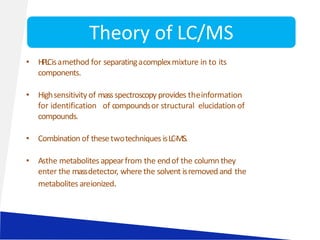
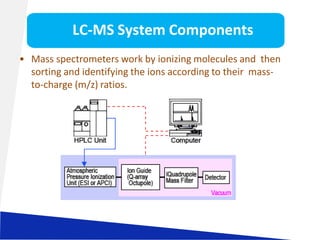
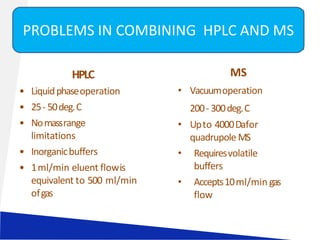
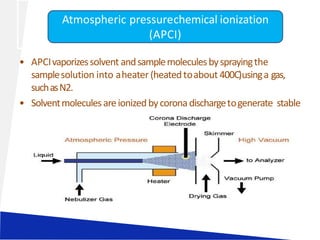
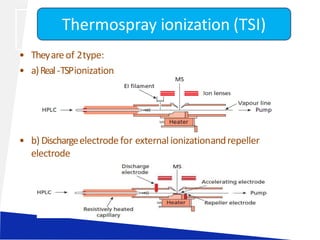
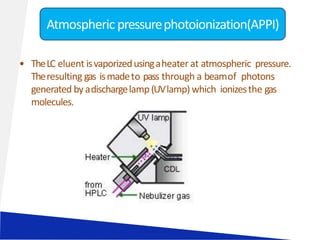
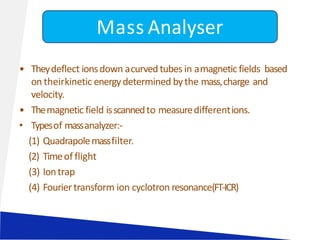
Liquid chromatography-mass spectrometry (LC-MS) is a technique that combines the separation capabilities of liquid chromatography (HPLC) with the detection capabilities of mass spectrometry, allowing for the identification and structural elucidation of compounds. Key components include interfaces for ionizing samples and various ion sources like electrospray and atmospheric pressure chemical ionization. LC-MS has diverse applications in pharmaceuticals, biochemistry, food safety, environmental monitoring, and forensics.




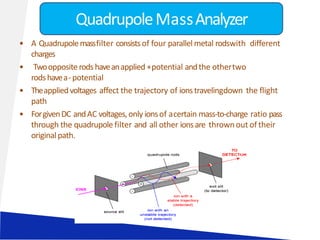

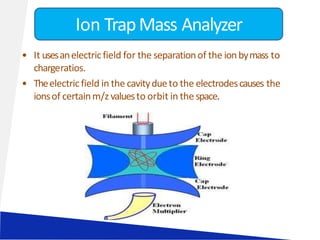
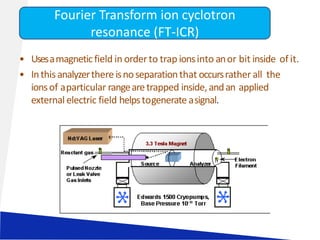


![LIQUID CHROMATOGRAPHY- MASS SPECTROSCOPY[LC-MS]](https://image.slidesharecdn.com/63-191219160156/85/LIQUID-CHROMATOGRAPHY-MASS-SPECTROSCOPY-LC-MS-23-320.jpg)