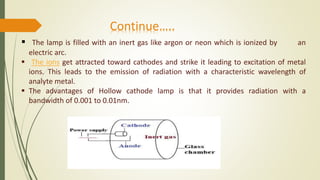
Atomic absorption spectroscopy is a common technique for detecting metals and metalloids in samples. It works by measuring the absorption of light by free metallic ions in a flame. The instrument uses a hollow cathode lamp to produce light of a specific wavelength, and a burner to atomize the liquid sample and produce free atoms. It can be used to quantitatively analyze over 62 elements in various materials like water, soil, biological samples, and ceramics. Some key applications include analyzing heavy metals in blood and urine, determining nutrient levels in foods, and measuring metal concentrations in groundwater.