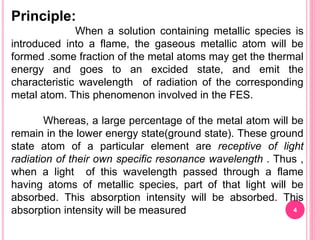
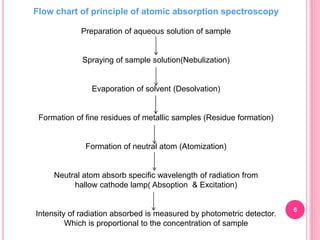
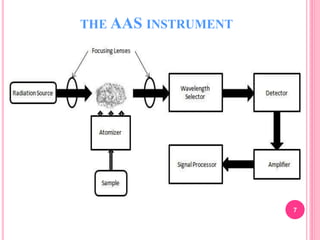
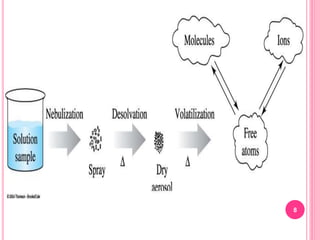
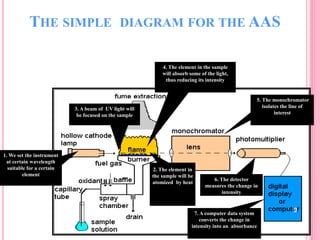
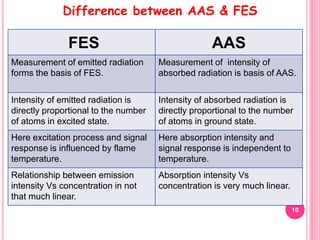
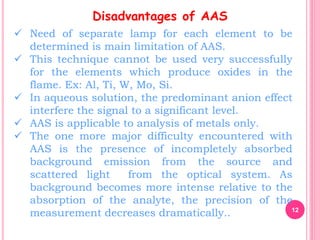
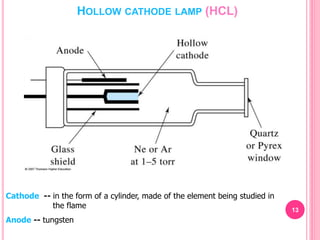
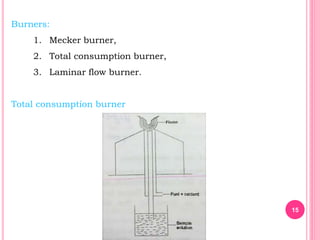
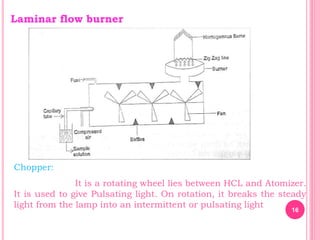
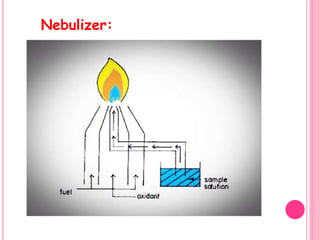
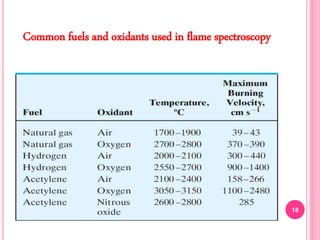
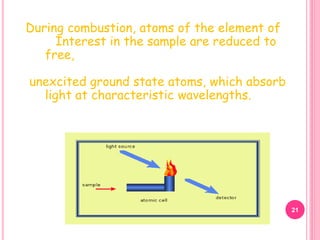
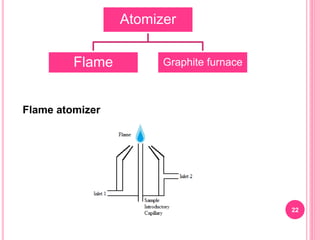
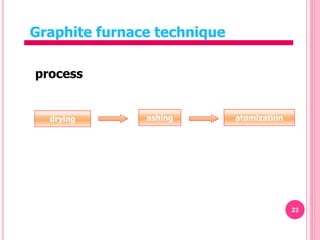
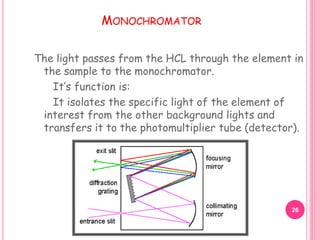
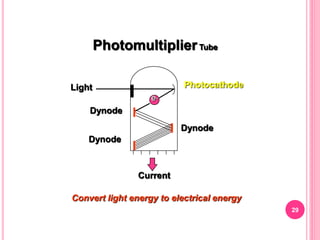
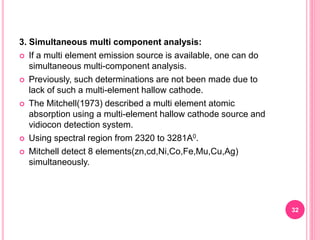
Atomic absorption spectroscopy is a quantitative analytical technique used to determine the concentration of metallic elements in a sample. It works by measuring the absorption of light passing through atoms of the element of interest that have been atomized in a flame. The amount of light absorbed is directly proportional to the number of ground state atoms present, which allows the technique to be used for quantitative analysis. The instrument consists of a hollow cathode lamp, burner, monochromator, and photomultiplier tube to measure absorption. Applications include determining trace elements in biological samples and quantifying metals in foods, soils, and industrial samples.