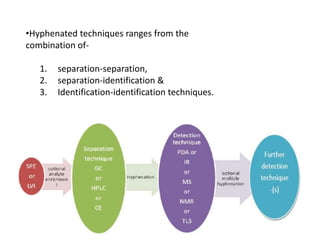
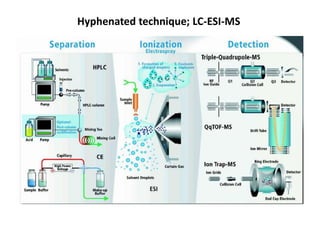
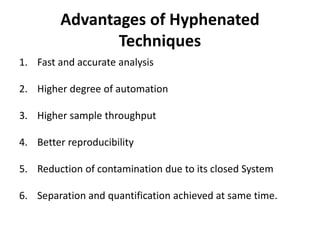
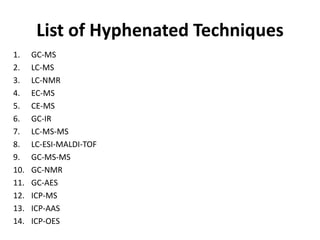
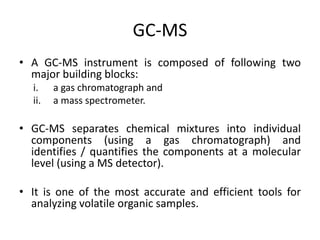
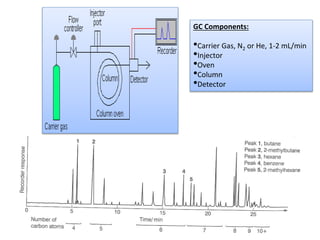
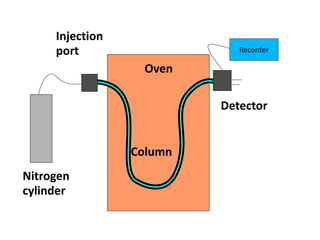
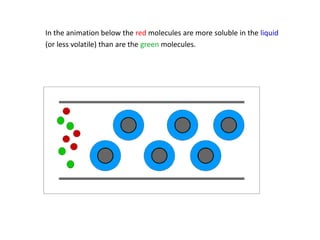
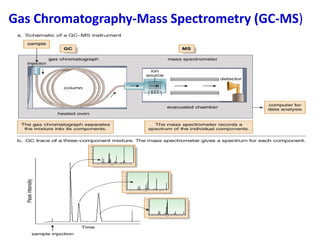
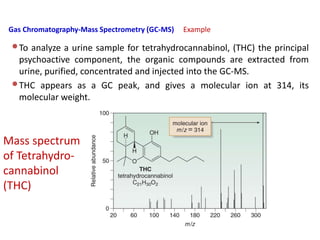
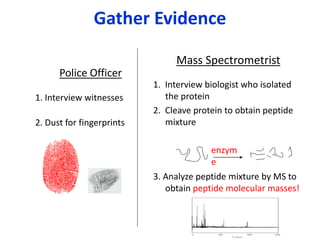
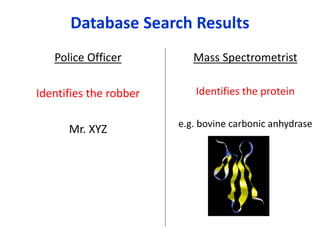
Analytical chemistry involves separating, identifying, and quantifying components of matter. Hyphenated techniques combine two analytical methods, such as gas chromatography coupled with mass spectrometry (GC-MS). GC-MS separates chemical mixtures using gas chromatography and then identifies components using mass spectrometry. Other common hyphenated techniques include liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-infrared spectroscopy (GC-IR). These coupled techniques provide enhanced sensitivity and accuracy for analyzing organic compounds, pollutants, drugs, proteins, and more.