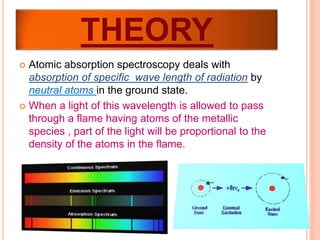
- Atomic absorption spectroscopy is a technique used to quantitatively determine trace metals in liquids. It works by vaporizing the liquid sample and measuring the absorption of light from hollow cathode lamps by atoms of the metal in the vapor.
- The amount of light absorbed is directly proportional to the number of metal atoms in the vapor. By measuring the absorption, the concentration of the metal in the original sample can be determined.
- Atomic absorption spectroscopy has detection limits in the parts-per-million or parts-per-billion range for many metals. It is a specific technique as each metal absorbs only its own characteristic wavelength.