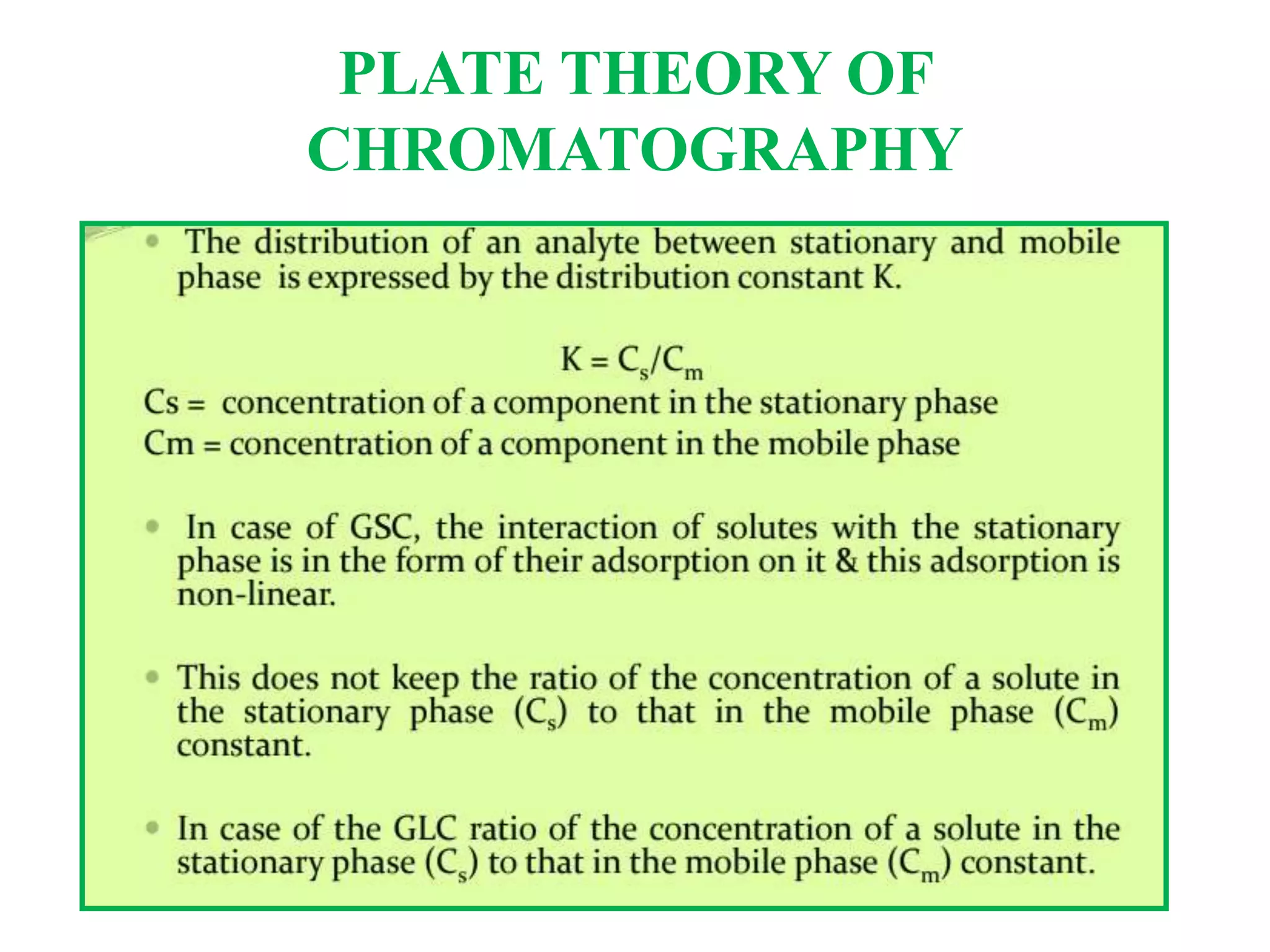
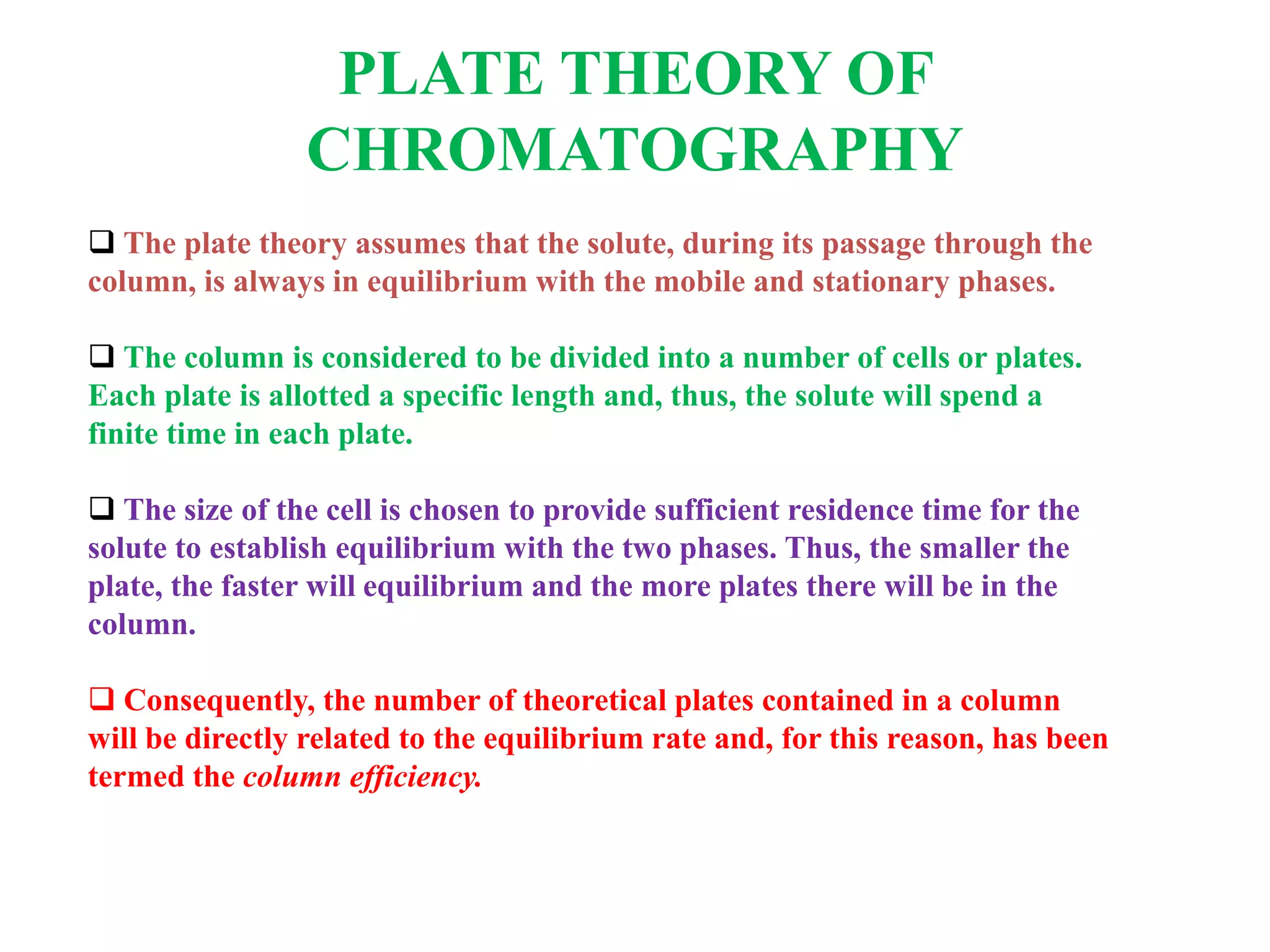
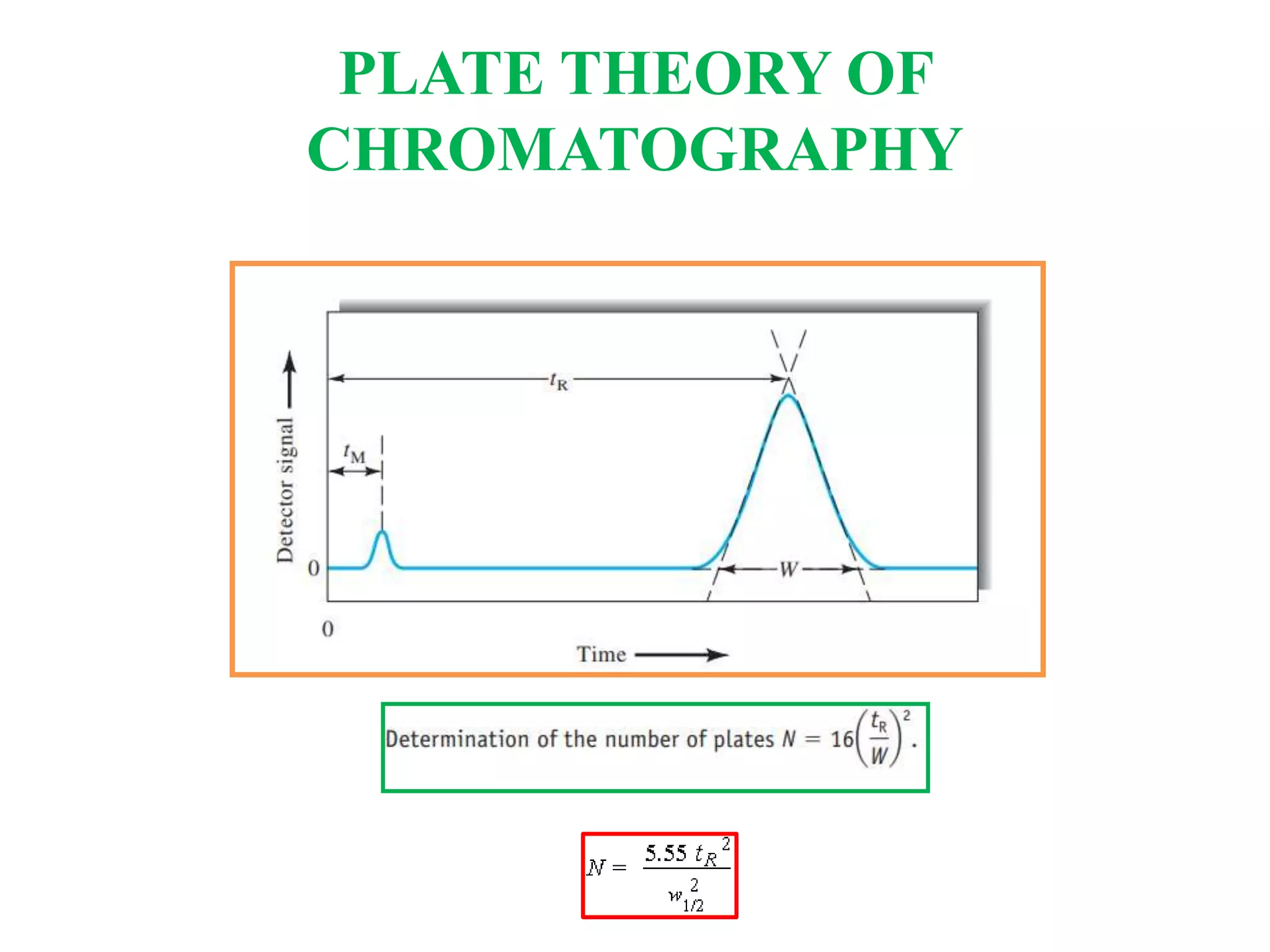
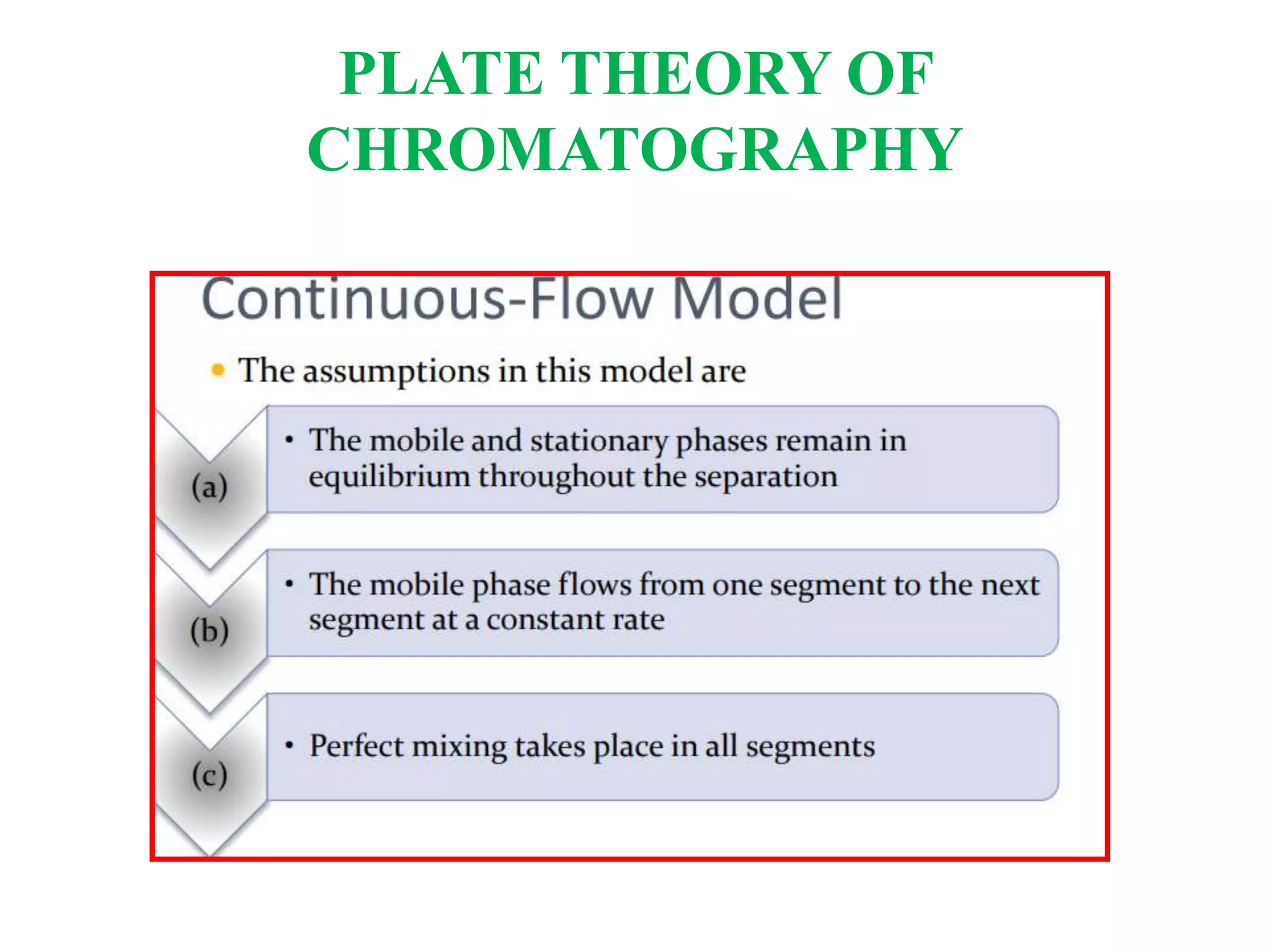
The Nobel Prize in Chemistry 1952 was awarded jointly to Archer John Porter Martin and Richard Laurence Millington Synge for their invention of partition chromatography. They developed the plate theory of chromatography, which models a chromatographic column as being divided into theoretical plates with each plate representing equilibrium between the mobile and stationary phases. The number of theoretical plates is used to represent the efficiency and performance of the column.

![Nernst Distribution law:
“At constant temperature, a solute
distributes itself between two immiscible
solvents only in a particular ratio”
KD = [X]SolventA
[X]SolventBPartition
Coefficient
PLATE THEORY OF
CHROMATOGRAPHY](https://image.slidesharecdn.com/platetheory-191123080052/75/Plate-theory-of-Chromatography-2-2048.jpg)