Embed presentation
Downloaded 139 times


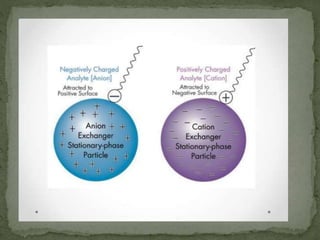

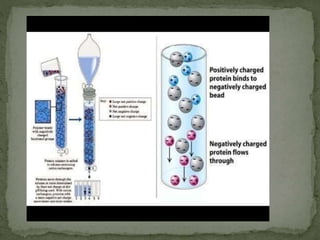
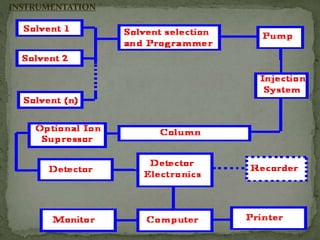




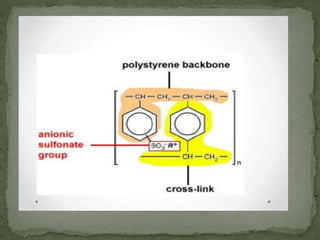








Ion-exchange chromatography separates molecules based on their charge through electrostatic interactions with an immobile matrix containing charged functional groups. It is utilized for applications such as deionization of water, purification of solutions, and separation of biomolecules and organic compounds. The technique is essential in clinical diagnostics and biochemical analysis for obtaining pure compounds.

















