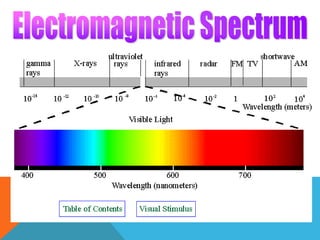
1. Atomic absorption spectroscopy involves exciting the electrons in metal atoms to higher energy levels using light from a hollow cathode lamp emitting a characteristic wavelength for the metal of interest.
2. When the excited metal atoms return to a lower energy state, they emit electromagnetic radiation of a specific wavelength that is measured by a detector.
3. The amount of light absorbed is directly proportional to the concentration of metal atoms, allowing the technique to be used for both qualitative and quantitative analysis of metals in solutions.