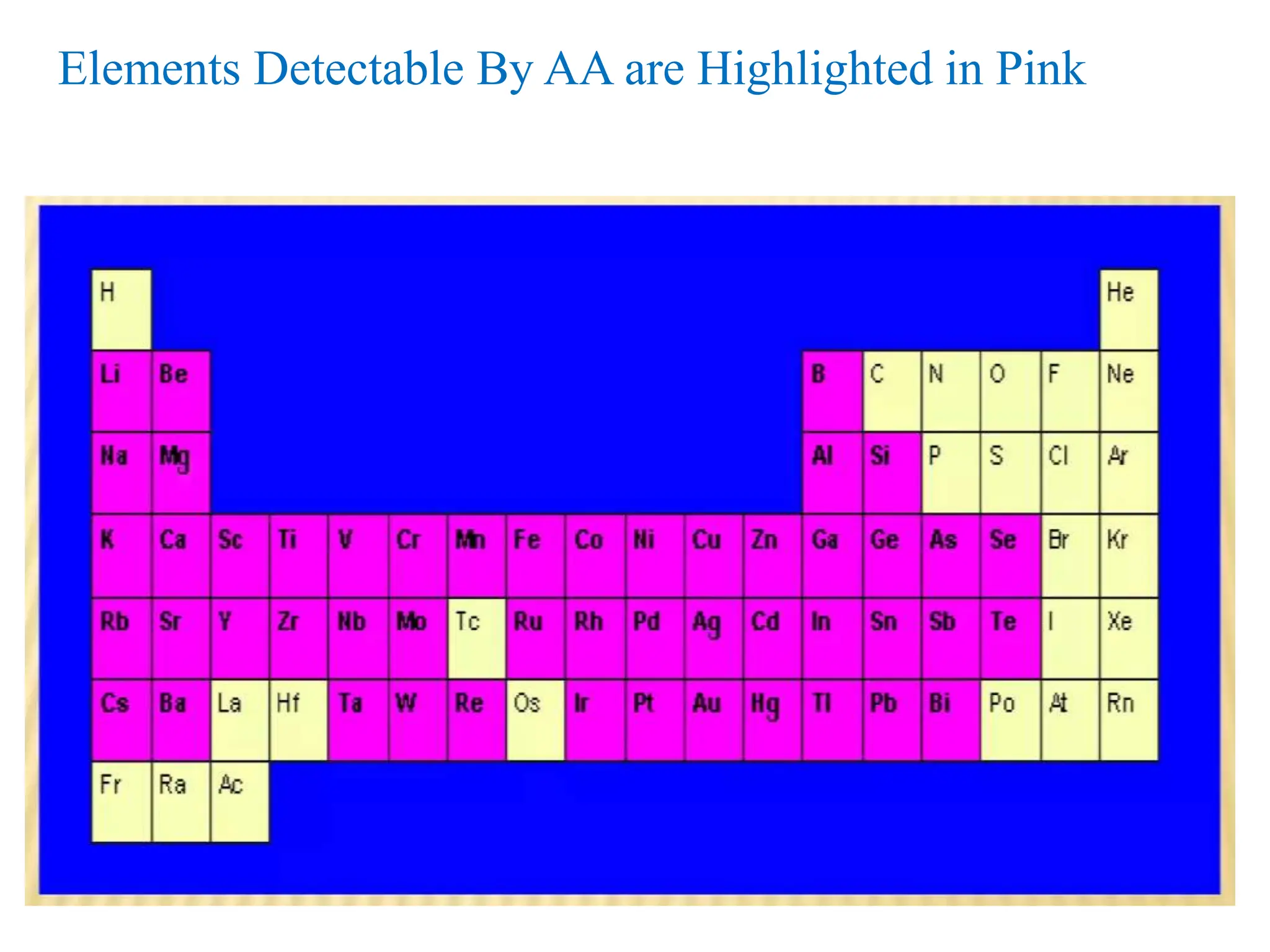
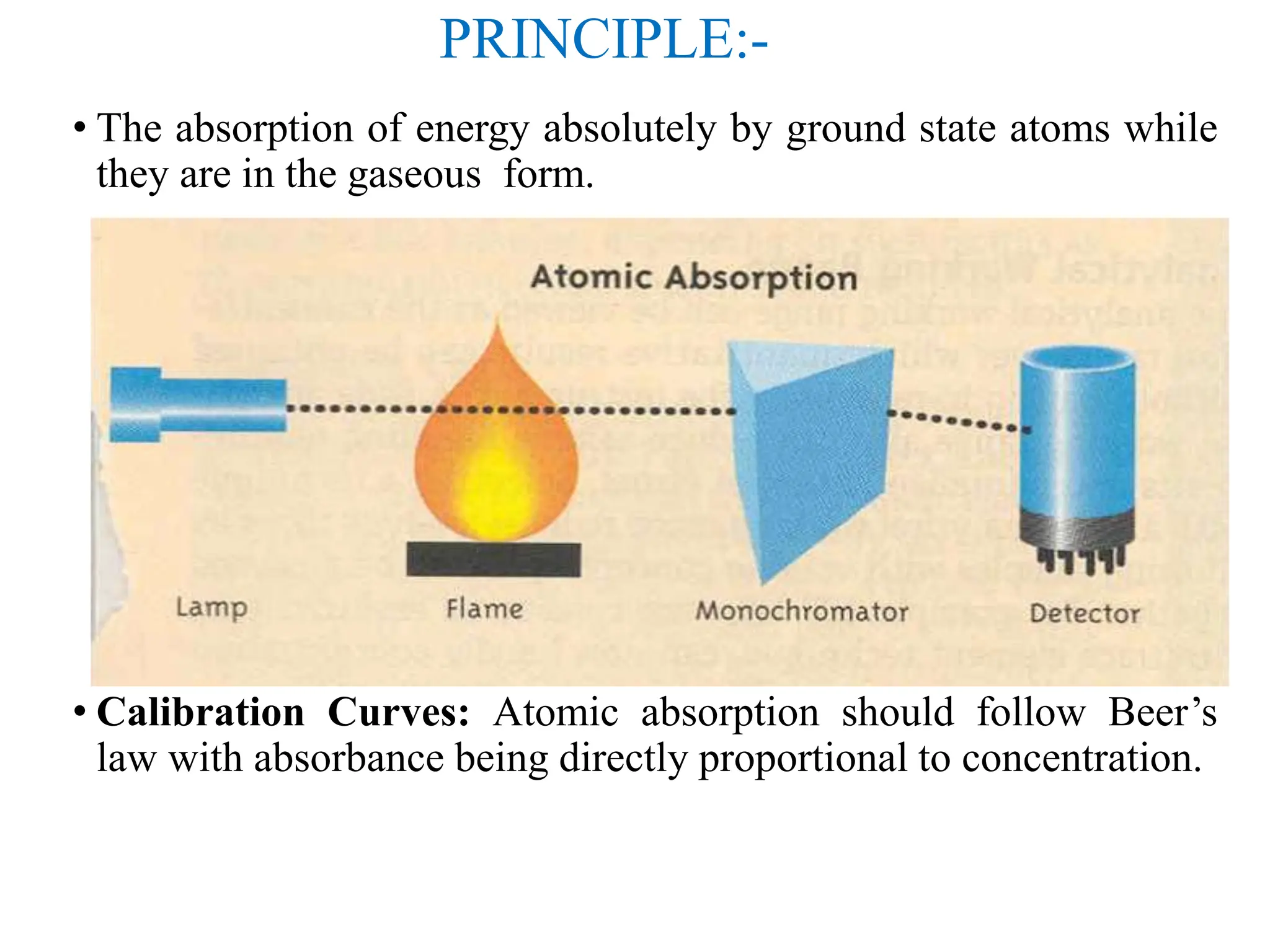
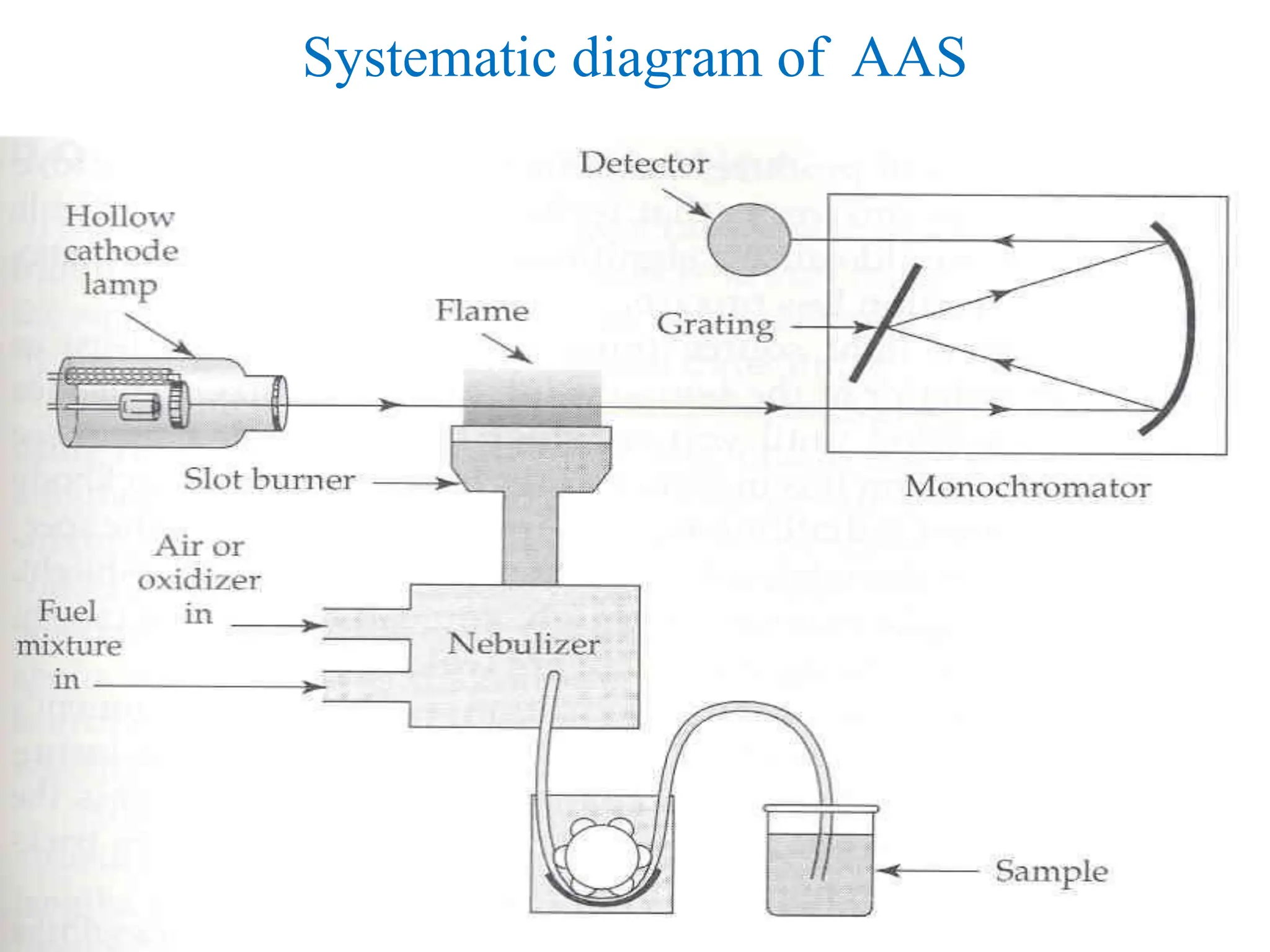
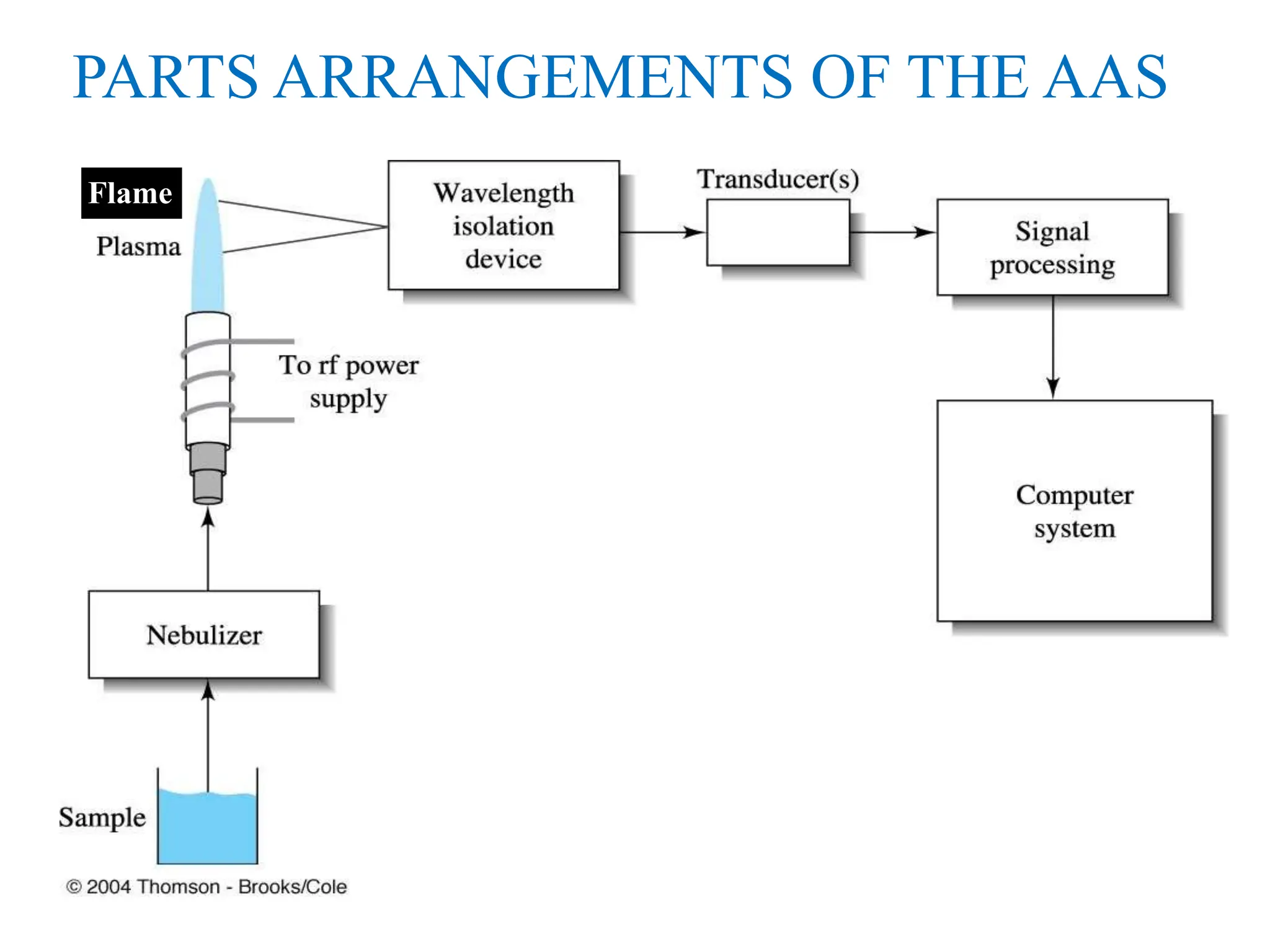
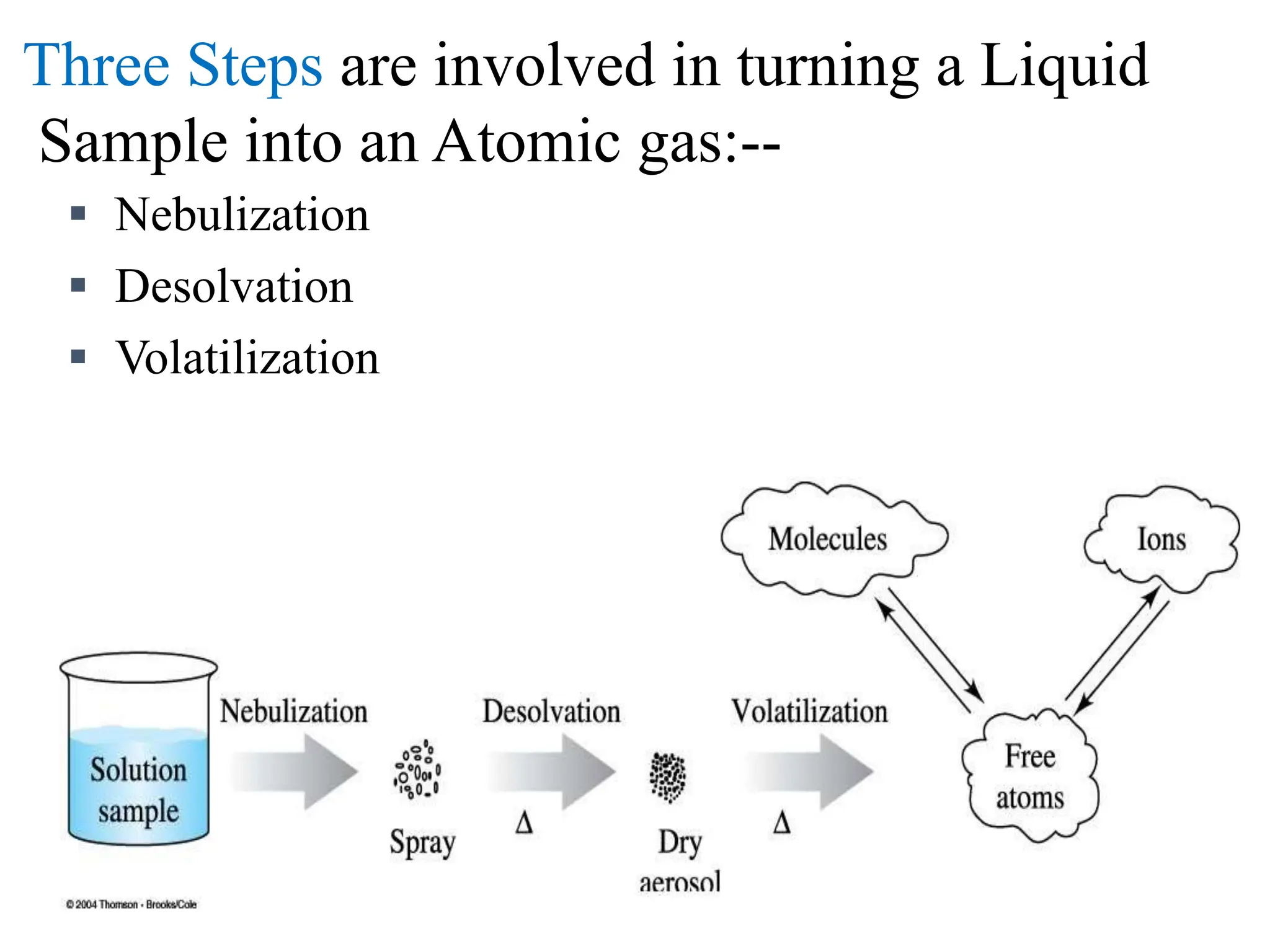
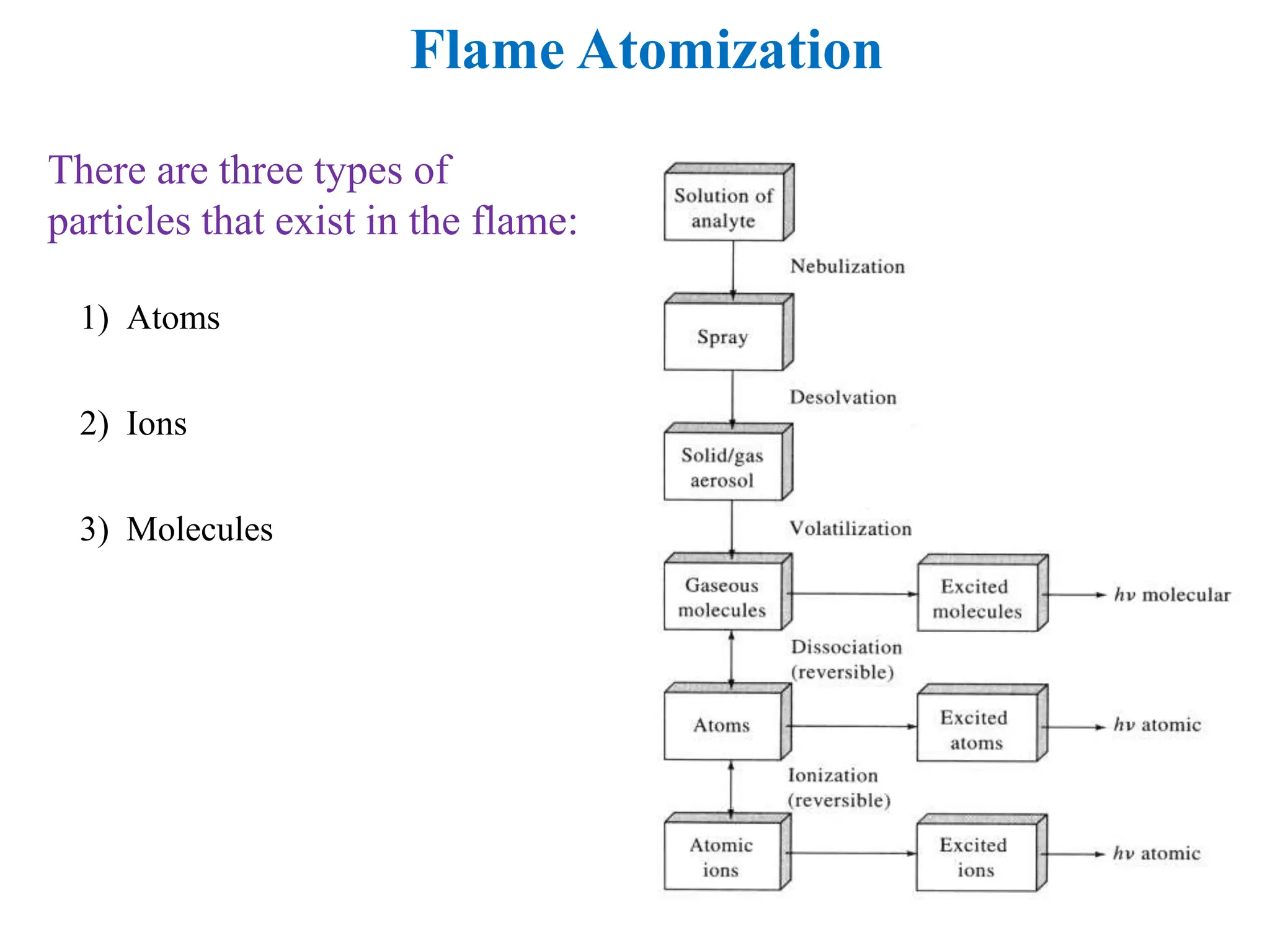
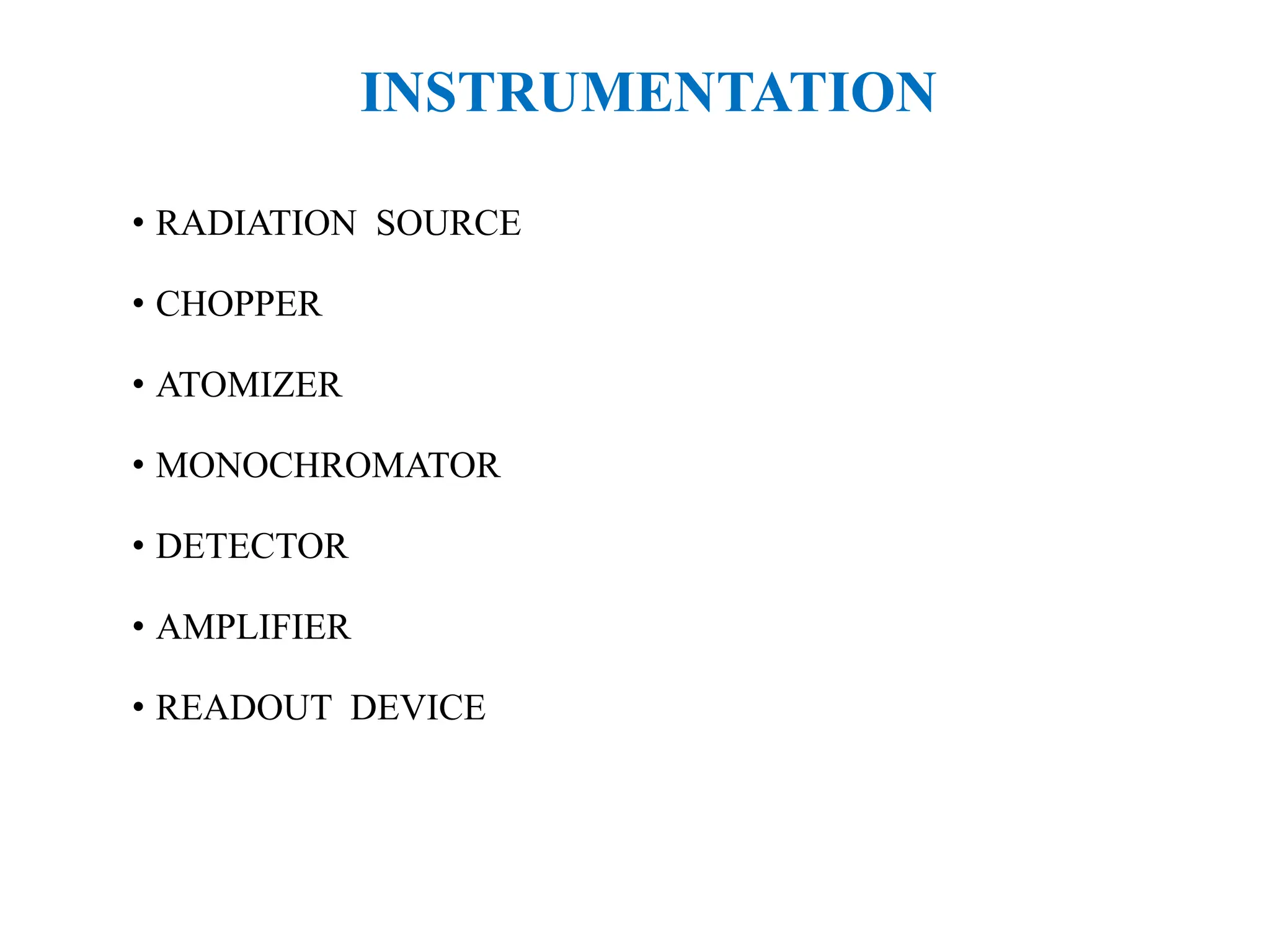
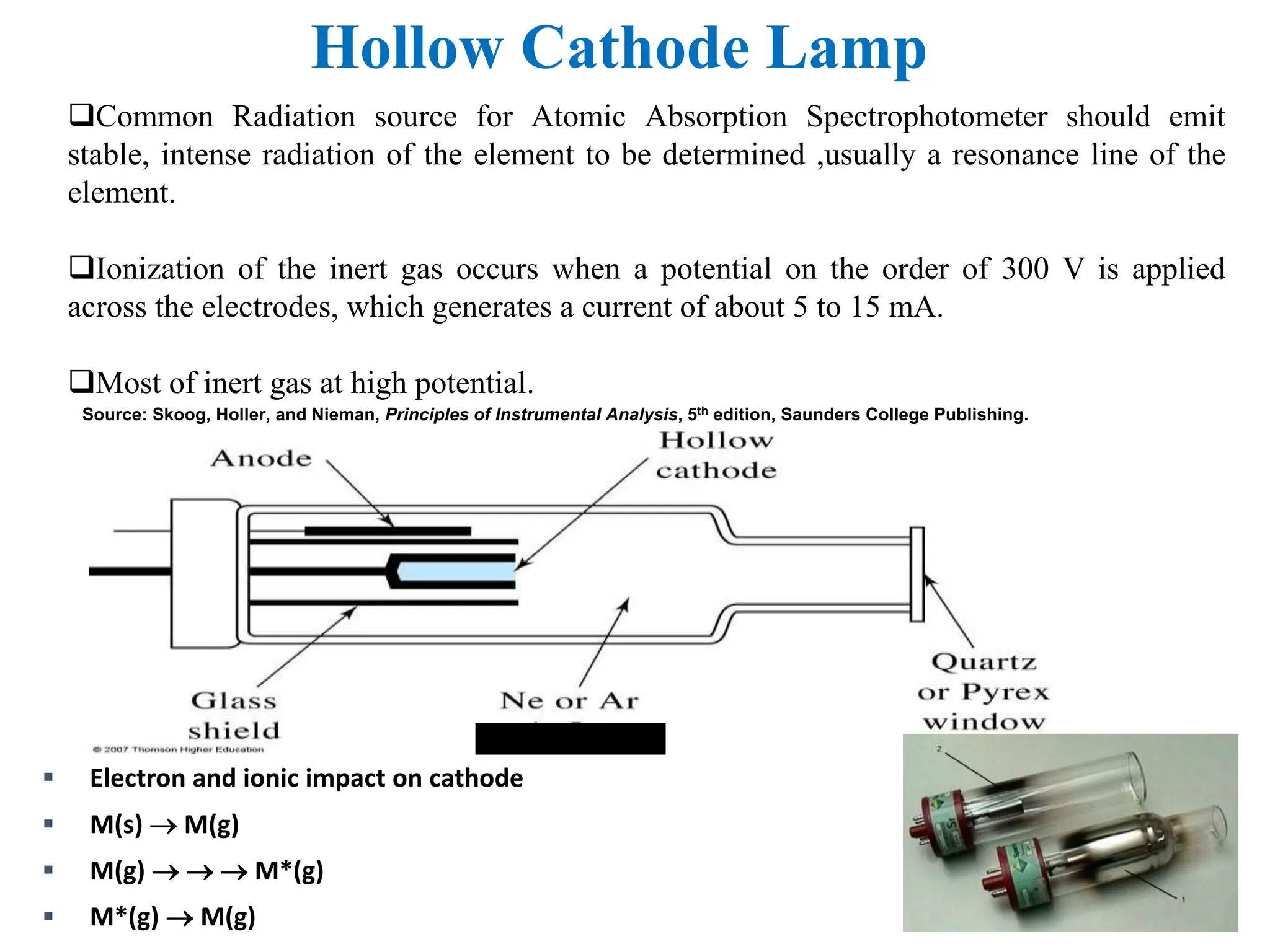
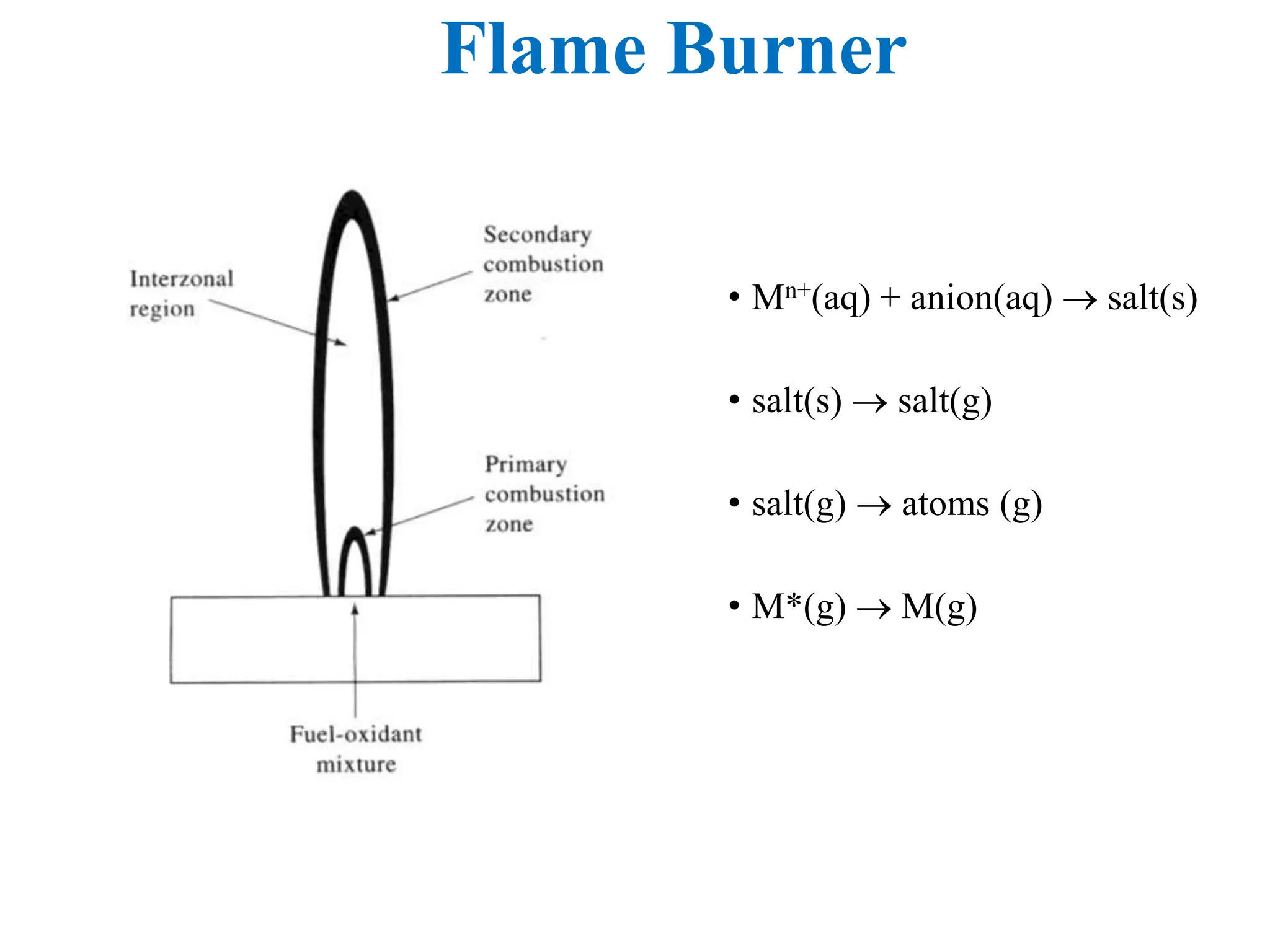
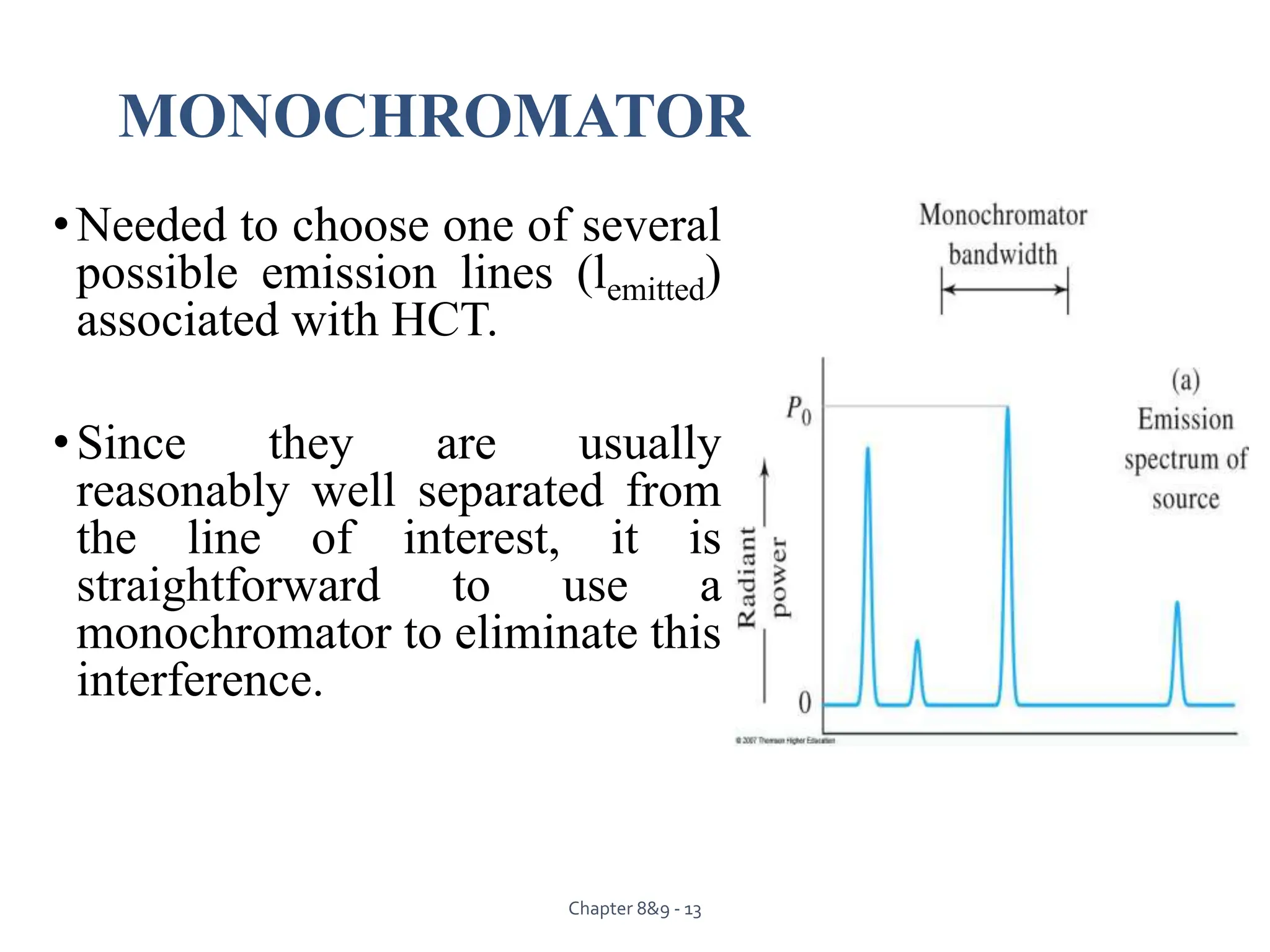
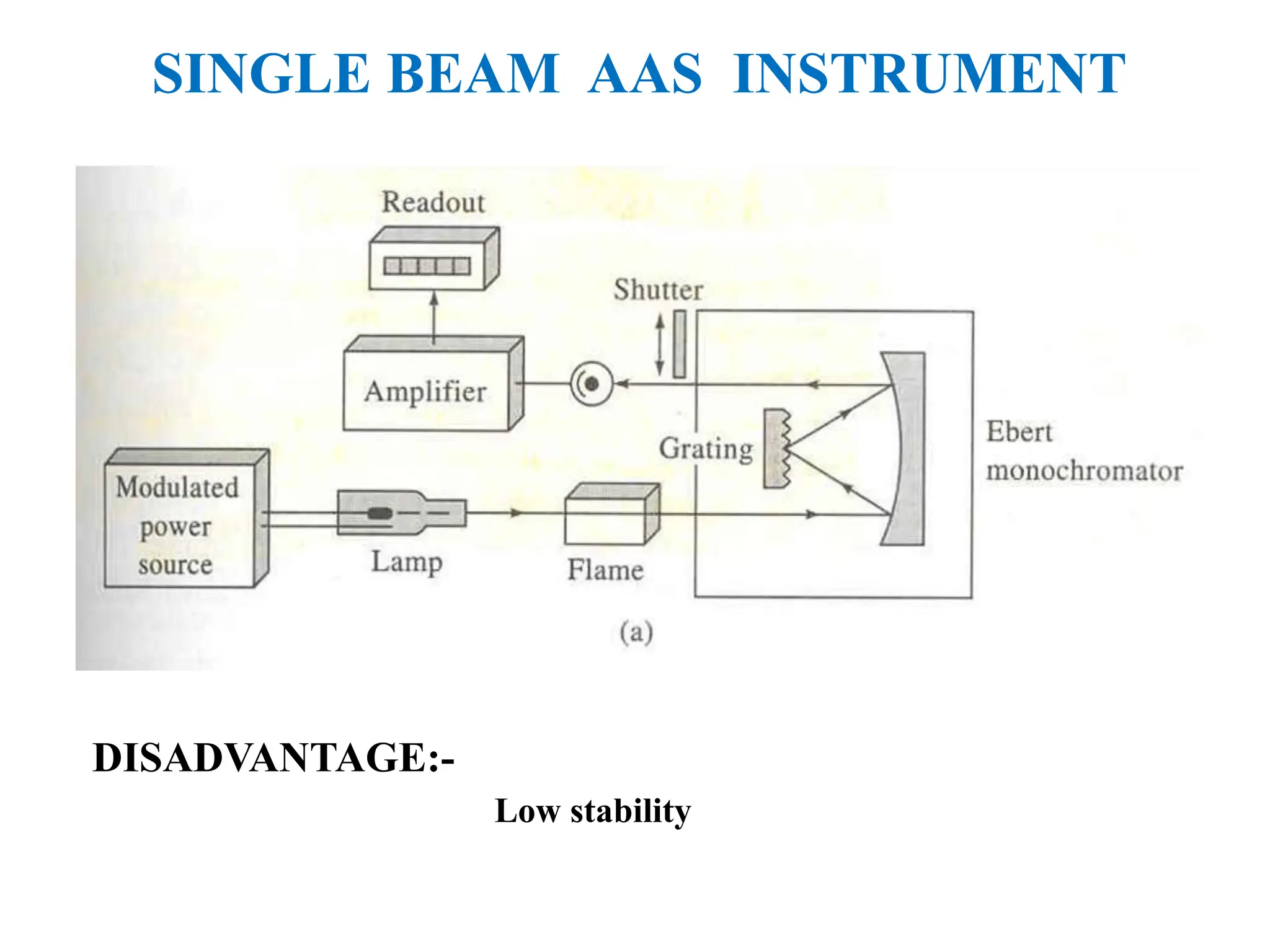
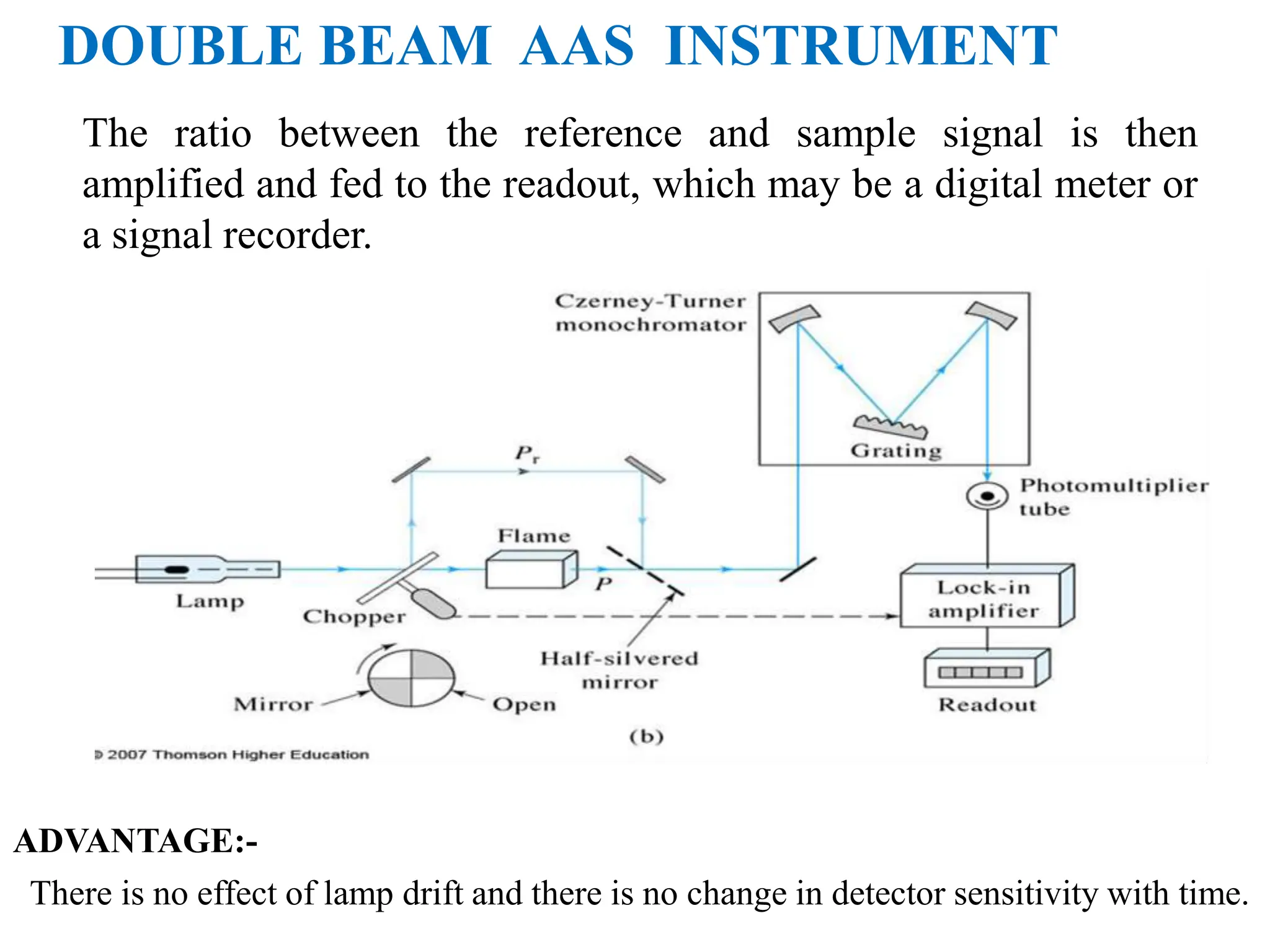
This document provides an overview of Atomic Absorption Spectroscopy (AAS), detailing its introduction, principles, instrumentation, advantages, disadvantages, and applications. AAS is a powerful analytical technique for determining trace metals in liquid samples, working on the basis of atomic absorption and requiring specific equipment. While it offers high precision and sensitivity, it is limited to certain elements and requires careful operation and sample preparation.