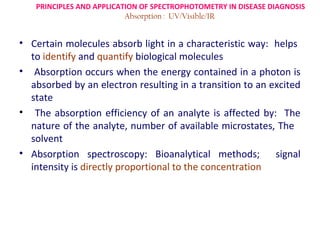
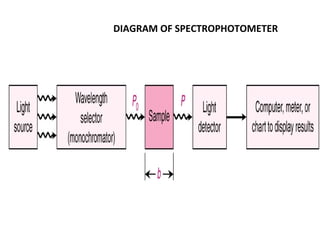
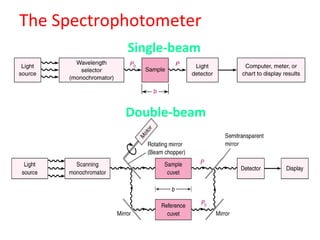
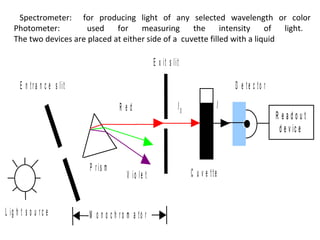
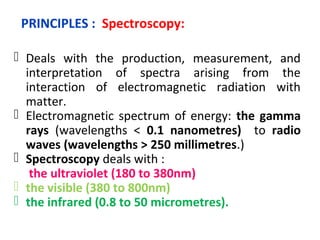
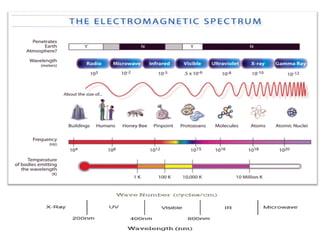
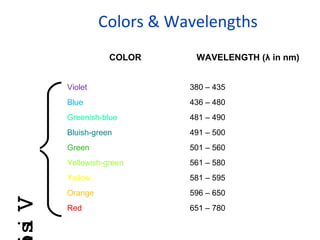
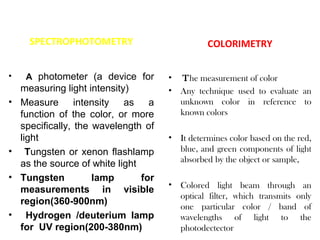
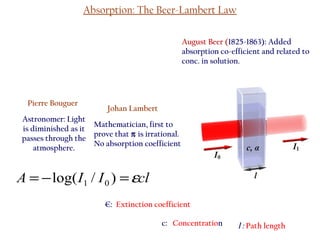
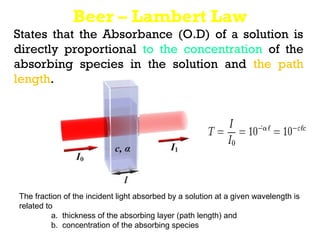
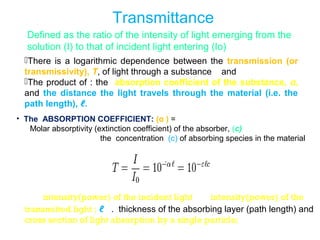
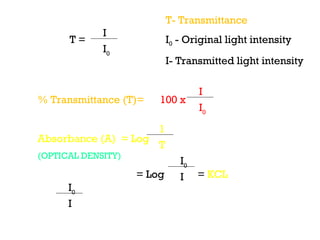
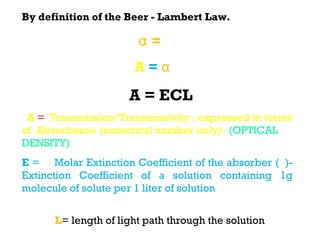
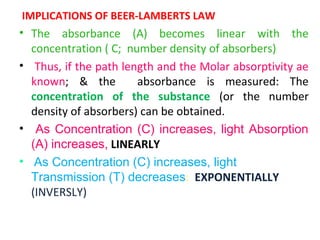
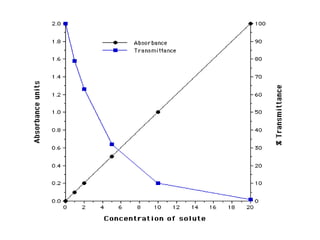
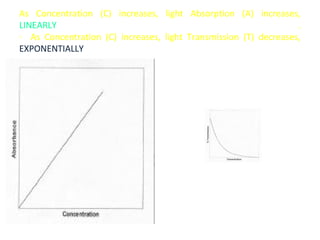
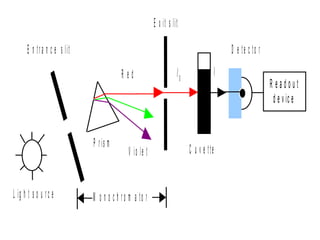
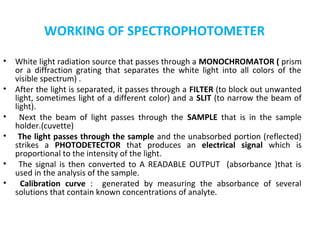
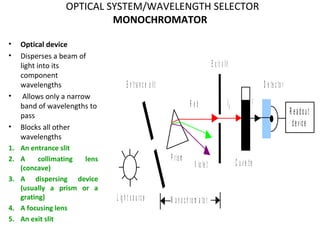
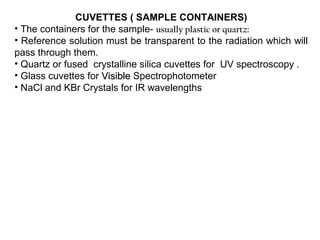
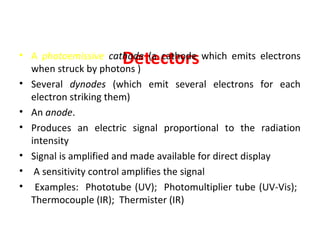
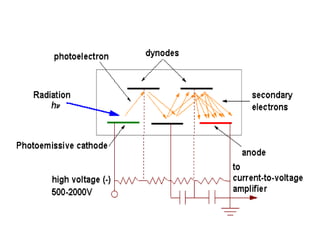
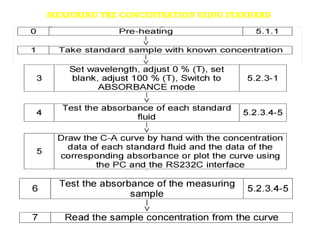
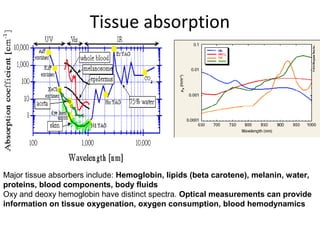
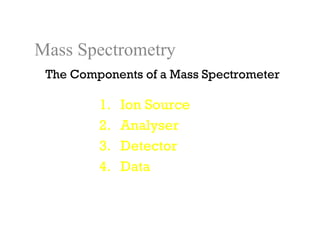
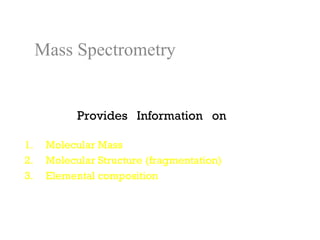
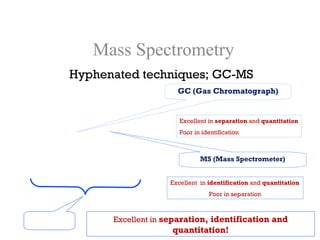
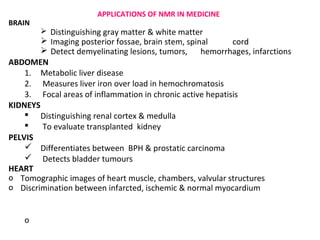
Spectrophotometry uses the principle that molecules absorb specific wavelengths of light. A spectrophotometer directs a beam of light through a sample and measures the amount of light absorbed. It contains a light source, wavelength selector like a prism or grating to produce monochromatic light, sample holders, a detector to measure transmitted light intensity, and a readout device. It works based on Beer's law, where absorbance is directly proportional to concentration, molar absorptivity, and path length. This allows spectrophotometry to quantify the concentration of an analyte by its optical properties.