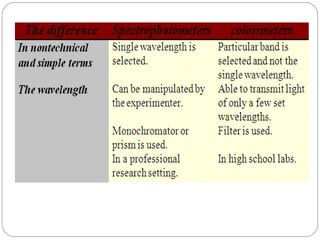
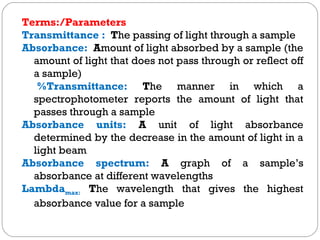
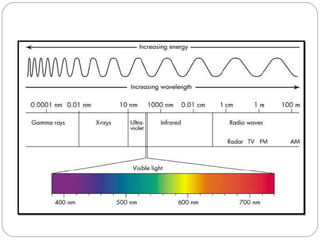
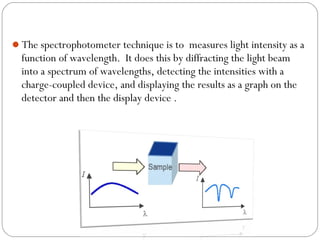
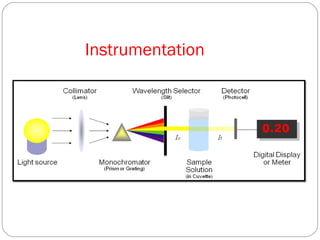
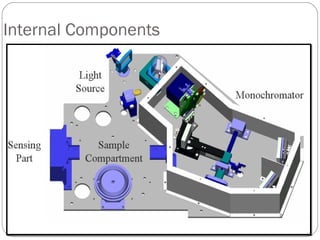
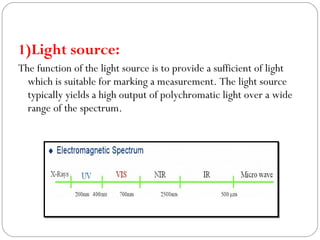
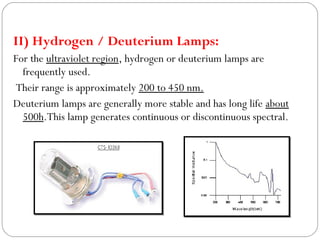
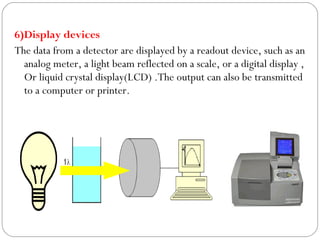
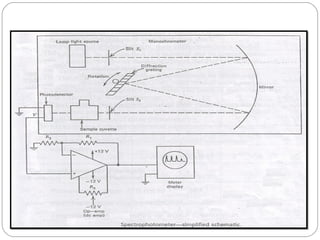
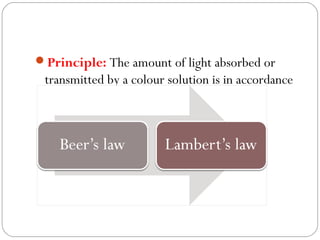
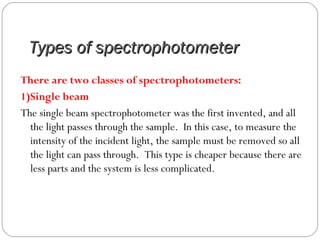
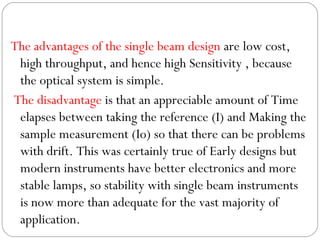
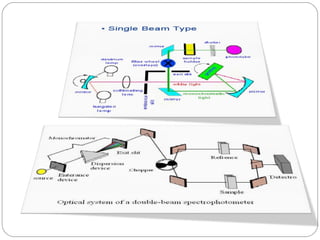
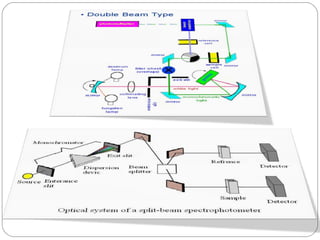
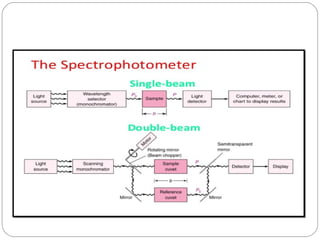
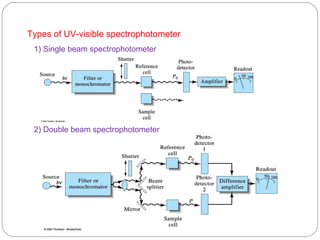
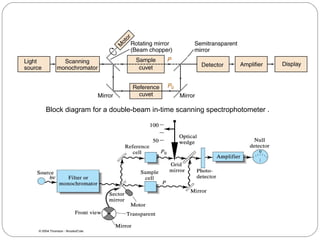
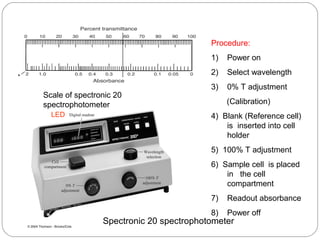
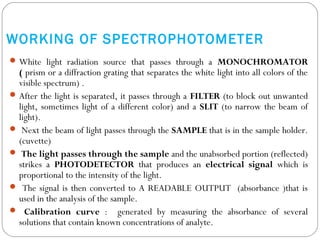
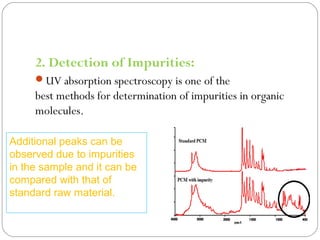
The document provides a comprehensive overview of spectrophotometers, detailing their definition, internal components, and various types. It explains how spectrophotometers function by measuring light absorbance in samples, including the roles of light sources, dispersion devices, and detectors. Additionally, the document outlines their applications in clinical diagnostics, impurity detection, and chemical kinetics.










![CONTD…
3. Structure elucidation of organic compounds.
From the location of peaks and combination of peaks
UV spectroscopy elucidate structure of organic molecules:
othe presence or absence of unsaturation,
othe presence of hetero atoms.[7]
45](https://image.slidesharecdn.com/6-150912091121-lva1-app6891/85/Spetrophotometer-45-320.jpg)