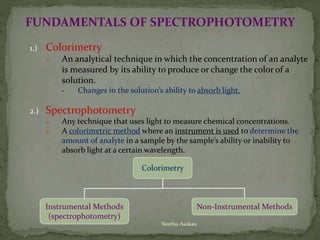
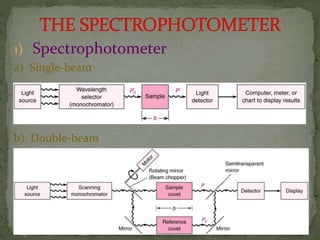
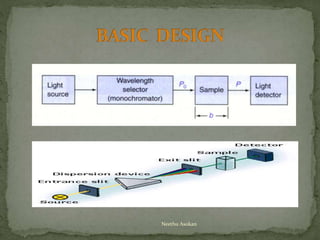
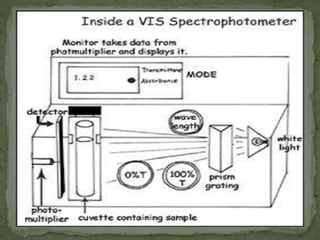
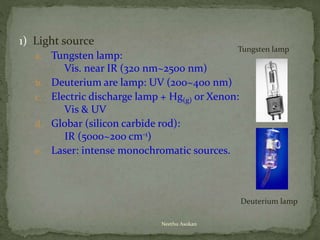
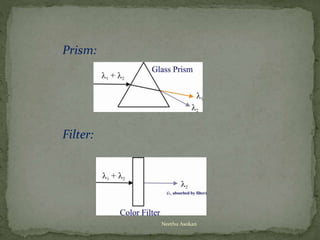
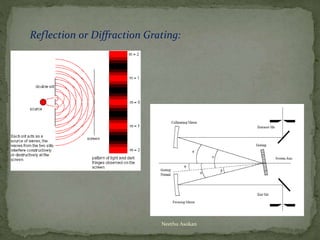
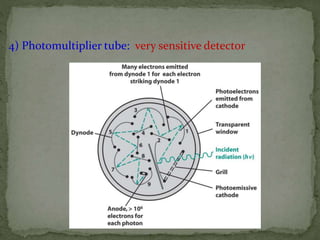
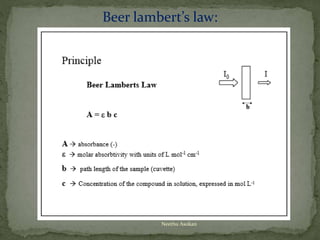
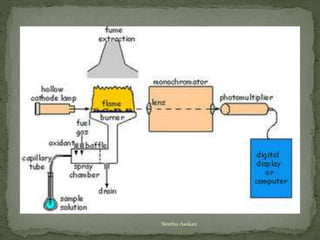
This document provides an overview of spectrophotometry and related analytical techniques. It defines colorimetry and spectrophotometry, describing spectrophotometry as a colorimetric method that uses an instrument to determine analyte concentration based on light absorption. Components of a spectrophotometer are described, including the light source, monochromator, cuvettes, detector, and components. Various types of spectrophotometers are discussed, as well as applications such as enzyme kinetics, organic stereochemistry studies, and more. Related techniques like fluorimetry, phosphorimetry, and circular dichroism are also summarized.